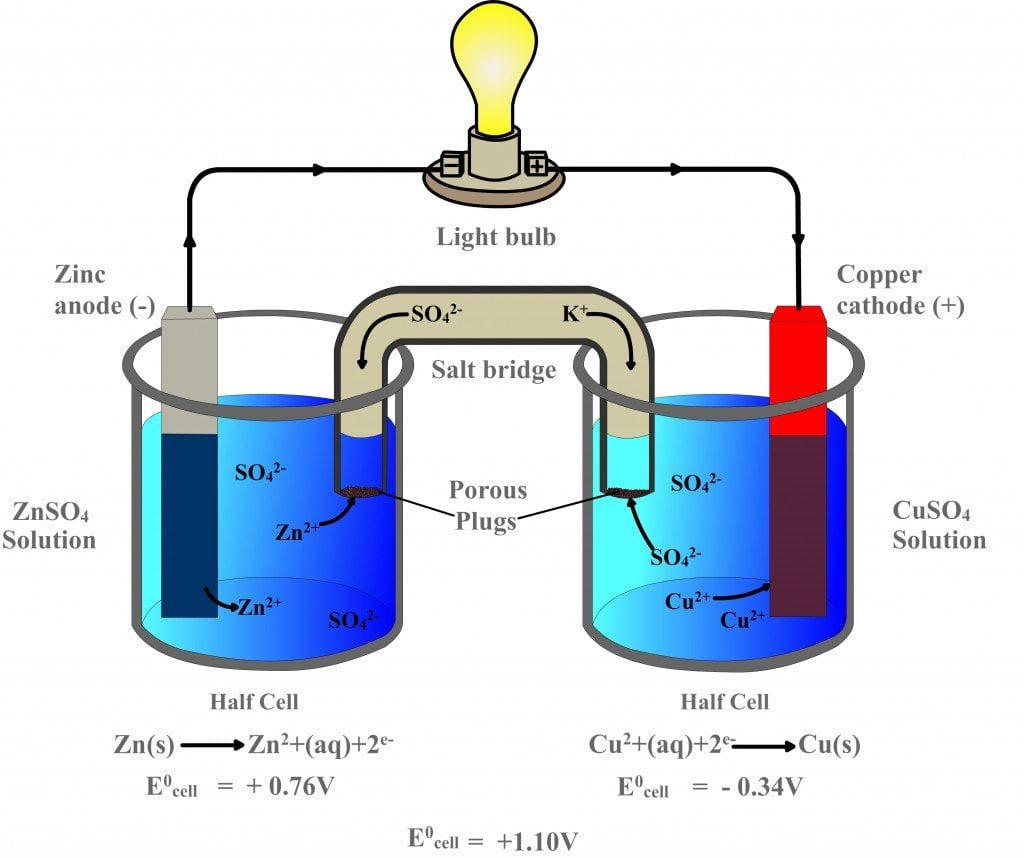
State The Difference(S) Between A Galvanic And An Electrolytic Cell . From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The main differences are outlined below: Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. However, there are also striking differences between the two cells. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical.
from www.scienceabc.com
A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. The main differences are outlined below: The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. However, there are also striking differences between the two cells. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a.
Galvanic Cell Definition, Diagram And Working
State The Difference(S) Between A Galvanic And An Electrolytic Cell A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. However, there are also striking differences between the two cells. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. The main differences are outlined below: Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell State The Difference(S) Between A Galvanic And An Electrolytic Cell From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. The main differences are outlined below: The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. This article explains the key differences between galvanic cell and electrolytic cell on the basis of. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From psiberg.com
Galvanic vs. Electrolytic Cell The Two Types of Electrochemical Cells State The Difference(S) Between A Galvanic And An Electrolytic Cell This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. The main differences are outlined below: The main distinction is that galvanic cells convert chemical energy into. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From exodrddcz.blob.core.windows.net
Difference Between Galvanic And Electrolytic Cell at Brian Chambless blog State The Difference(S) Between A Galvanic And An Electrolytic Cell Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. The main. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From fixlibrarymarkbladgr.z13.web.core.windows.net
Draw A Labelled Diagram Of Electrolytic Cell State The Difference(S) Between A Galvanic And An Electrolytic Cell However, there are also striking differences between the two cells. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. The main differences are outlined below: A galvanic cell is an electrochemical. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content State The Difference(S) Between A Galvanic And An Electrolytic Cell A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The main differences are outlined below: Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion,. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From worksheetfullrusskies.z21.web.core.windows.net
Diagram Of Electrochemical Cell State The Difference(S) Between A Galvanic And An Electrolytic Cell This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From differguide.com
Difference Between Galvanic And Electrolytic Cell State The Difference(S) Between A Galvanic And An Electrolytic Cell Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. However, there are also striking differences between the two cells. From the above differences between galvanic and electrolytic cells, we can conclude that. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From www.differencebetween.com
Difference Between Galvanic Cell and Concentration Cell Compare the State The Difference(S) Between A Galvanic And An Electrolytic Cell The main differences are outlined below: Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion,. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell State The Difference(S) Between A Galvanic And An Electrolytic Cell The main differences are outlined below: However, there are also striking differences between the two cells. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate electrical energy. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From schoolbag.info
Electrochemistry Review the Knowledge You Need to Score High 5 State The Difference(S) Between A Galvanic And An Electrolytic Cell The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. A galvanic cell. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From www.differencebetween.net
Difference Between Galvanic Cells and Electrolytic Cells Difference State The Difference(S) Between A Galvanic And An Electrolytic Cell A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. The main differences are outlined below: This article explains the key differences between galvanic cell and electrolytic cell on the basis. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From www.aiophotoz.com
The Difference Between Galvanic Cells And Electrolytic Cells Easychem State The Difference(S) Between A Galvanic And An Electrolytic Cell The main differences are outlined below: However, there are also striking differences between the two cells. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. From the above differences between. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From pediaa.com
Difference Between Galvanic and Electrolytic Cell Definition, How State The Difference(S) Between A Galvanic And An Electrolytic Cell Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. However, there are also striking differences between the two cells. The main differences are outlined below: This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. The main distinction is. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From allthedifferences.com
What Is The Difference Between Electrolytic Cells And Galvanic Cells State The Difference(S) Between A Galvanic And An Electrolytic Cell This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. However, there are also striking differences between the two cells. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic cell is an electrochemical cell in which spontaneous redox. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From www.vrogue.co
Difference Between Galvanic Cells And Electrolytic Ce vrogue.co State The Difference(S) Between A Galvanic And An Electrolytic Cell This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. However, there are also striking differences. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From www.pinterest.es
What are the differences between Galvanic cell and Electrolytic cell State The Difference(S) Between A Galvanic And An Electrolytic Cell Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. However, there are also striking differences between the two cells. The main differences are outlined below: Galvanic cells generate electrical. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell State The Difference(S) Between A Galvanic And An Electrolytic Cell The main differences are outlined below: Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. From the above differences between galvanic and electrolytic cells, we can conclude that a. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From general.chemistrysteps.com
Electrolytic Cells Chemistry Steps State The Difference(S) Between A Galvanic And An Electrolytic Cell Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. However, there are also striking differences between the two cells. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. This article explains the key differences between galvanic cell and. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From exodrddcz.blob.core.windows.net
Difference Between Galvanic And Electrolytic Cell at Brian Chambless blog State The Difference(S) Between A Galvanic And An Electrolytic Cell Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. The main differences are outlined below: From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells State The Difference(S) Between A Galvanic And An Electrolytic Cell A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. Electrolytic cells are primarily used for. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From www.differencebetween.com
What is the Difference Between Voltaic Cell and Electrolytic Cell State The Difference(S) Between A Galvanic And An Electrolytic Cell Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. However, there are also striking differences between the two cells. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From study.com
Voltaic vs. Electrolytic Cell Similarities, Differences & Uses State The Difference(S) Between A Galvanic And An Electrolytic Cell However, there are also striking differences between the two cells. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Electrolytic cells are primarily used for processes that require the. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From www.vrogue.co
What Is The Difference Between Galvanic Cell And Elec vrogue.co State The Difference(S) Between A Galvanic And An Electrolytic Cell Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. The main differences are outlined below: Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell State The Difference(S) Between A Galvanic And An Electrolytic Cell The main differences are outlined below: However, there are also striking differences between the two cells. The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. A galvanic cell is an electrochemical cell in which. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From exodrddcz.blob.core.windows.net
Difference Between Galvanic And Electrolytic Cell at Brian Chambless blog State The Difference(S) Between A Galvanic And An Electrolytic Cell However, there are also striking differences between the two cells. The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. This article explains the key differences between galvanic cell and electrolytic cell. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From www.youtube.com
DIFFERENCES BETWEEN GALVANIC & ELECTROLYTIC CELL YouTube State The Difference(S) Between A Galvanic And An Electrolytic Cell The main differences are outlined below: However, there are also striking differences between the two cells. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. Electrolytic. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From socratic.org
The following cell is operated as an electrolytic cell, using a current State The Difference(S) Between A Galvanic And An Electrolytic Cell Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. The main. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From www.javatpoint.com
Difference Between Galvanic Cells and Electrolytic Cells javatpoint State The Difference(S) Between A Galvanic And An Electrolytic Cell The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. The main differences are outlined below: Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki State The Difference(S) Between A Galvanic And An Electrolytic Cell The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. This article explains the. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From socratic.org
How can a galvanic cell an electrolytic cell? Socratic State The Difference(S) Between A Galvanic And An Electrolytic Cell Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. However, there are also striking differences between the two cells. This article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox reaction, polarity,. Electrolytic cells are primarily used for processes that require the input. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From hxeapbuuh.blob.core.windows.net
What Is Galvanic Cell And Electrolytic Cell at John Pool blog State The Difference(S) Between A Galvanic And An Electrolytic Cell Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. However, there are also striking differences between the two cells. This article explains the key differences between galvanic cell and electrolytic cell on the. State The Difference(S) Between A Galvanic And An Electrolytic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working State The Difference(S) Between A Galvanic And An Electrolytic Cell Electrolytic cells are primarily used for processes that require the input of energy, such as electroplating and chemical synthesis. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. However, there are also striking differences between the two cells. The main distinction is that galvanic cells convert chemical energy into electrical energy,. State The Difference(S) Between A Galvanic And An Electrolytic Cell.