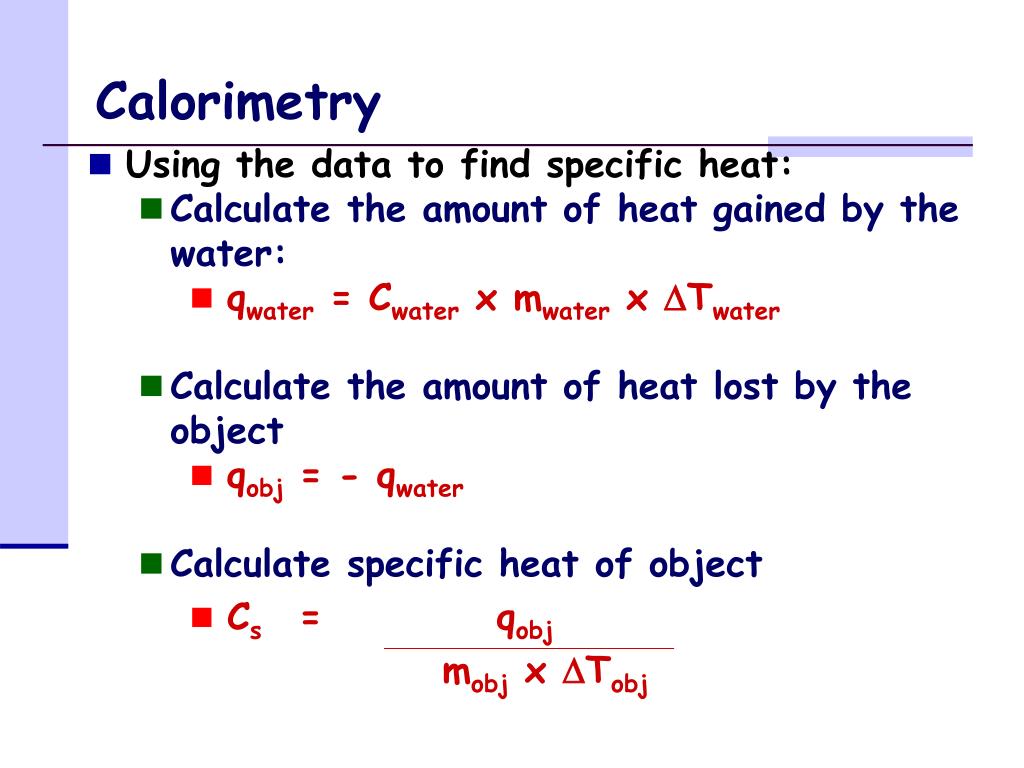
Calorimeter Formula Sheet . Use the equation for heat transfer \(q =. It is made up of thin sheet. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Compare and contrast coffee cup calorimetry and bomb calorimetry. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. the formula for calorimetry: Where, solved examples for calorimetry formula. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Qsub = m × c × δt. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. it is a vessel to measure the amount of heat gained or lost by a body when mixed with another body. A physics class has been assigned. Calorimetry defines the act of measuring different changes in state variables of a body for deriving the heat. Calorimetry is a fascinating branch of thermodynamics that enables us to. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry.
from www.slideserve.com
Use the equation for heat transfer \(q =. Qsub = m × c × δt. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Calorimetry is a fascinating branch of thermodynamics that enables us to. Compare and contrast coffee cup calorimetry and bomb calorimetry. the formula for calorimetry: A physics class has been assigned. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter.
PPT Calorimetry PowerPoint Presentation, free download ID6133898
Calorimeter Formula Sheet Qsub = m × c × δt. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. the formula for calorimetry: Compare and contrast coffee cup calorimetry and bomb calorimetry. Calorimetry defines the act of measuring different changes in state variables of a body for deriving the heat. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Where, solved examples for calorimetry formula. Use the equation for heat transfer \(q =. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. it is a vessel to measure the amount of heat gained or lost by a body when mixed with another body. Qsub = m × c × δt. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A physics class has been assigned. It is made up of thin sheet. Calorimetry is a fascinating branch of thermodynamics that enables us to. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimeter Formula Sheet A physics class has been assigned. it is a vessel to measure the amount of heat gained or lost by a body when mixed with another body. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a container that prevents heat transfer in or out is called a. Calorimeter Formula Sheet.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Calorimeter Formula Sheet it is a vessel to measure the amount of heat gained or lost by a body when mixed with another body. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. Where, solved examples for calorimetry formula. the formula for. Calorimeter Formula Sheet.
From www.studypool.com
SOLUTION Physics calorimetry formula notes Studypool Calorimeter Formula Sheet Calorimetry defines the act of measuring different changes in state variables of a body for deriving the heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It is made up of thin sheet. the formula for calorimetry: it is a vessel to measure the. Calorimeter Formula Sheet.
From www.chegg.com
Solved DATA SHEET (Calorimetry] Table 1 Calorimeter Calorimeter Formula Sheet One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry defines the act of measuring different changes in state variables of a body for deriving the heat. Use the equation for heat transfer \(q =. Calculate the molar heat of enthalpy for a reactions using coffee cup. Calorimeter Formula Sheet.
From www.scribd.com
calorimetry Calorimeter Formula Sheet Calorimetry defines the act of measuring different changes in state variables of a body for deriving the heat. It is made up of thin sheet. A physics class has been assigned. Where, solved examples for calorimetry formula. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. . Calorimeter Formula Sheet.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube Calorimeter Formula Sheet Use the equation for heat transfer \(q =. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. the formula for calorimetry: Compare and contrast coffee cup calorimetry and bomb calorimetry. It is made up of thin sheet. Where, solved examples for calorimetry formula. Calorimetry is a. Calorimeter Formula Sheet.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimeter Formula Sheet the formula for calorimetry: A physics class has been assigned. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry defines the act of measuring different changes in state variables of a body for deriving the heat. Use the equation for heat transfer \(q =. One technique we can. Calorimeter Formula Sheet.
From exyqpgrbs.blob.core.windows.net
Calorimetry Equation Explained at Sandra Roof blog Calorimeter Formula Sheet A physics class has been assigned. Qsub = m × c × δt. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. the formula for calorimetry: Compare and contrast coffee cup calorimetry and bomb calorimetry. Calorimetry is a fascinating branch of thermodynamics that enables us to. it is. Calorimeter Formula Sheet.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Formula Sheet a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Qsub = m × c × δt. Use the equation for heat transfer \(q =. Calorimetry is a fascinating branch of thermodynamics that enables us to. Calorimetry defines the act of measuring different changes in state variables. Calorimeter Formula Sheet.
From www.scribd.com
Phy CH 8 Formula Sheet Elasticity, Thermal Expansion, Calorimetry PDF Calorimeter Formula Sheet Calorimetry defines the act of measuring different changes in state variables of a body for deriving the heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter. Calorimeter Formula Sheet.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimeter Formula Sheet Use the equation for heat transfer \(q =. the formula for calorimetry: a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Qsub = m × c × δt. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in. Calorimeter Formula Sheet.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimeter Formula Sheet Compare and contrast coffee cup calorimetry and bomb calorimetry. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. the formula for calorimetry: a container that prevents. Calorimeter Formula Sheet.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimeter Formula Sheet Use the equation for heat transfer \(q =. A physics class has been assigned. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. the formula for calorimetry: Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. a container that prevents heat. Calorimeter Formula Sheet.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Formula Sheet Calorimetry defines the act of measuring different changes in state variables of a body for deriving the heat. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Qsub = m × c × δt. a container that prevents heat transfer in or out is called a. Calorimeter Formula Sheet.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimeter Formula Sheet One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. the. Calorimeter Formula Sheet.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Calorimeter Formula Sheet A physics class has been assigned. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Compare and contrast coffee cup calorimetry and bomb calorimetry. it is a vessel to measure the amount of heat gained or lost by a body when mixed with another body. . Calorimeter Formula Sheet.
From exyxwauhc.blob.core.windows.net
What Is Calorimetry And How Does It Work at Jesse Mignone blog Calorimeter Formula Sheet Qsub = m × c × δt. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calorimetry is a fascinating branch of thermodynamics that enables us to. A physics class has been assigned. calculate heat, temperature. Calorimeter Formula Sheet.
From www.youtube.com
Calorimetry Part 2 calculations YouTube Calorimeter Formula Sheet the formula for calorimetry: Compare and contrast coffee cup calorimetry and bomb calorimetry. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Calorimetry is a fascinating branch of thermodynamics that enables us to. it is a vessel to measure the amount of heat gained or. Calorimeter Formula Sheet.
From www.scribd.com
Calorimetry Formula Sheet PDF Calorimeter Formula Sheet Calorimetry defines the act of measuring different changes in state variables of a body for deriving the heat. It is made up of thin sheet. Where, solved examples for calorimetry formula. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. A physics class has been assigned. Calorimetry. Calorimeter Formula Sheet.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimeter Formula Sheet Calorimetry defines the act of measuring different changes in state variables of a body for deriving the heat. A physics class has been assigned. Qsub = m × c × δt. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calorimetry is a fascinating branch of thermodynamics that enables us to. Use the equation for heat. Calorimeter Formula Sheet.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 Calorimeter Formula Sheet calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calorimetry is a fascinating branch of thermodynamics that enables us to. Qsub = m × c × δt. Calorimetry defines the act of measuring different changes in state variables of a body. Calorimeter Formula Sheet.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimeter Formula Sheet Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare and contrast. Calorimeter Formula Sheet.
From classlibrarycordeiro.z21.web.core.windows.net
Calorimetry Calculations Worksheet Chart Calorimeter Formula Sheet calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. It is made up of thin sheet. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Where, solved examples for calorimetry formula. Calorimetry defines the act of. Calorimeter Formula Sheet.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter Formula Sheet Qsub = m × c × δt. Use the equation for heat transfer \(q =. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. it is a vessel to measure the amount of heat gained or lost by a body when mixed with another body.. Calorimeter Formula Sheet.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Formula Sheet Compare and contrast coffee cup calorimetry and bomb calorimetry. it is a vessel to measure the amount of heat gained or lost by a body when mixed with another body. A physics class has been assigned. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. calculate heat, temperature change, and specific heat after thermal. Calorimeter Formula Sheet.
From www.studocu.com
Calorimetry CALORIMETRY Report sheet A. Determination of the Calorimeter Formula Sheet Calorimetry defines the act of measuring different changes in state variables of a body for deriving the heat. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. Calorimeter Formula Sheet.
From www.chegg.com
Solved Calculate Heat capacity of the calorimeter (J/ ºC) , Calorimeter Formula Sheet It is made up of thin sheet. Calorimetry defines the act of measuring different changes in state variables of a body for deriving the heat. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. calculate heat, temperature change, and specific heat after thermal equilibrium is reached. Calorimeter Formula Sheet.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimeter Formula Sheet the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. the formula for calorimetry: a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Calculate the molar heat of enthalpy for a reactions using. Calorimeter Formula Sheet.
From revisionug.com
What is Calorimetry? Calorimeter Formula Sheet the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Where, solved examples for calorimetry formula. it is a vessel to measure the amount of heat gained or lost by a body when mixed with another body. the formula for calorimetry: Qsub = m × c. Calorimeter Formula Sheet.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimeter Formula Sheet a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It is made up of thin sheet. the formula for calorimetry: Calorimetry defines the. Calorimeter Formula Sheet.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter Formula Sheet Calorimetry is a fascinating branch of thermodynamics that enables us to. it is a vessel to measure the amount of heat gained or lost by a body when mixed with another body. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A physics class has been assigned. Calculate the. Calorimeter Formula Sheet.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter Formula Sheet Calorimetry is a fascinating branch of thermodynamics that enables us to. It is made up of thin sheet. A physics class has been assigned. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare and contrast coffee cup calorimetry and bomb calorimetry. it is a vessel. Calorimeter Formula Sheet.
From exywsebyp.blob.core.windows.net
Calorimetry Equation Physics at Charles Marshall blog Calorimeter Formula Sheet Qsub = m × c × δt. Where, solved examples for calorimetry formula. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Calorimetry defines the act of measuring different changes in state variables. Calorimeter Formula Sheet.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimeter Formula Sheet the formula for calorimetry: the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Where, solved examples for calorimetry formula. Calorimetry defines the act. Calorimeter Formula Sheet.
From exyqpgrbs.blob.core.windows.net
Calorimetry Equation Explained at Sandra Roof blog Calorimeter Formula Sheet the formula for calorimetry: Calorimetry is a fascinating branch of thermodynamics that enables us to. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Qsub = m × c × δt. One technique we can use to measure the amount of heat involved in a. Calorimeter Formula Sheet.