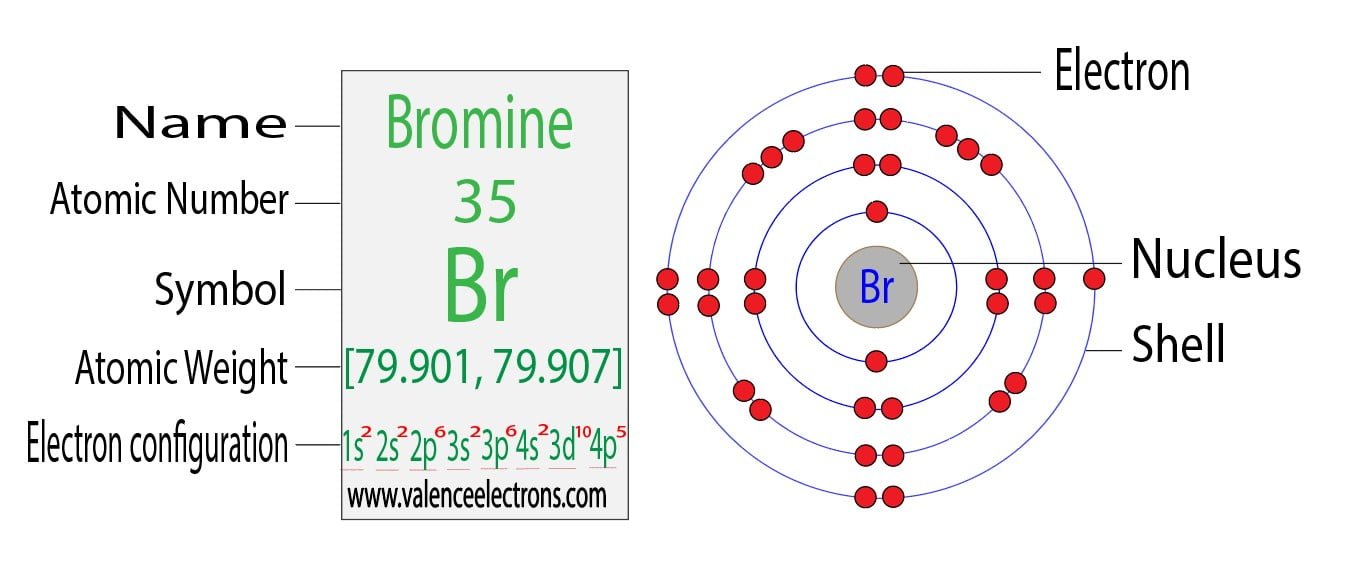
Does Bromine Want To Gain Or Lose Electrons . elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. this table shows the most common charges for atoms of the chemical elements. The atomic number for bromine is 35, which means it has 35 protons in its. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble. an atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of. in general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion.
from giomwhfig.blob.core.windows.net
an atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of. The atomic number for bromine is 35, which means it has 35 protons in its. determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble. elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. in general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. this table shows the most common charges for atoms of the chemical elements.
Bromine Equation Electron at Alexis Barnhart blog
Does Bromine Want To Gain Or Lose Electrons this table shows the most common charges for atoms of the chemical elements. an atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. in general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. this table shows the most common charges for atoms of the chemical elements. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. The atomic number for bromine is 35, which means it has 35 protons in its. determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble.
From socratic.org
How can you tell if an element wants to gain or lose electrons? Socratic Does Bromine Want To Gain Or Lose Electrons The atomic number for bromine is 35, which means it has 35 protons in its. determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron. Does Bromine Want To Gain Or Lose Electrons.
From www.slideserve.com
PPT UNIT 1 Structure and Function (Biochemistry) Chapter 2 Does Bromine Want To Gain Or Lose Electrons elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the. Does Bromine Want To Gain Or Lose Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Does Bromine Want To Gain Or Lose Electrons elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. an atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of. determine the number of electrons bromine needs to gain or lose to achieve the. Does Bromine Want To Gain Or Lose Electrons.
From www.slideshare.net
10 28 How Many Electrons Do Atoms Gain Lose Does Bromine Want To Gain Or Lose Electrons in general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. an atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of. The atomic number for bromine is 35, which means it has 35 protons in. Does Bromine Want To Gain Or Lose Electrons.
From giomwhfig.blob.core.windows.net
Bromine Equation Electron at Alexis Barnhart blog Does Bromine Want To Gain Or Lose Electrons electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. in general, metals will lose electrons to become a positive cation and nonmetals. Does Bromine Want To Gain Or Lose Electrons.
From hxehgfslt.blob.core.windows.net
Does Bromine And Sulfur Form An Ionic Compound at Charlie Wright blog Does Bromine Want To Gain Or Lose Electrons determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble. in general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. The atomic number for bromine is 35, which means it has 35 protons in its. . Does Bromine Want To Gain Or Lose Electrons.
From www.teachoo.com
Elements P, Q, R & S have atomic numbers 11, 15, 17 & 18 respectively Does Bromine Want To Gain Or Lose Electrons in general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. determine the number of electrons bromine needs to gain or lose to. Does Bromine Want To Gain Or Lose Electrons.
From www.numerade.com
SOLVED 0 / 1 pts Question 11 As per octet rule, bromine atom would Does Bromine Want To Gain Or Lose Electrons this table shows the most common charges for atoms of the chemical elements. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. The atomic number for bromine is 35, which means it has 35 protons in its. elements in other groups have partially filled valence shells. Does Bromine Want To Gain Or Lose Electrons.
From slideplayer.com
Unit 5 Ionic Bonding & Nomenclature ppt download Does Bromine Want To Gain Or Lose Electrons elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. this table shows the most common charges for atoms of the chemical elements. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added.. Does Bromine Want To Gain Or Lose Electrons.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Does Bromine Want To Gain Or Lose Electrons in general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. an atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of. this table shows the most common charges for atoms of the chemical elements.. Does Bromine Want To Gain Or Lose Electrons.
From www.slideshare.net
Understanding The Chemistry Of Atoms to Ions Does Bromine Want To Gain Or Lose Electrons elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. The atomic number for bromine is 35, which means it has 35 protons in its. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. an atom. Does Bromine Want To Gain Or Lose Electrons.
From www.youtube.com
Valence Electrons Gaining and Losing Electrons YouTube Does Bromine Want To Gain Or Lose Electrons electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. The atomic number for bromine is 35, which means it has 35 protons in. Does Bromine Want To Gain Or Lose Electrons.
From www.slideshare.net
Bonding Basics Does Bromine Want To Gain Or Lose Electrons elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. determine the number of electrons bromine needs to gain or lose to achieve. Does Bromine Want To Gain Or Lose Electrons.
From studylib.net
By GAINING or LOSING electrons Does Bromine Want To Gain Or Lose Electrons this table shows the most common charges for atoms of the chemical elements. elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added.. Does Bromine Want To Gain Or Lose Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Does Bromine Want To Gain Or Lose Electrons elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. in general, metals will lose electrons to become a positive cation and nonmetals. Does Bromine Want To Gain Or Lose Electrons.
From www.coursehero.com
[Solved] Neutral bromine (Br2) can gain two electrons to the Does Bromine Want To Gain Or Lose Electrons in general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. an atom of bromine in the gas phase, for example, gives off. Does Bromine Want To Gain Or Lose Electrons.
From h-o-m-e.org
Bromide (Br) Ion Charges Explained Does Bromine Want To Gain Or Lose Electrons in general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble. an atom of bromine in the gas phase, for example, gives off energy when it. Does Bromine Want To Gain Or Lose Electrons.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Does Bromine Want To Gain Or Lose Electrons electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. determine the number of electrons bromine needs to gain or lose to achieve. Does Bromine Want To Gain Or Lose Electrons.
From utedzz.blogspot.com
Periodic Table Electrons Lost Or Gained Periodic Table Timeline Does Bromine Want To Gain Or Lose Electrons atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. in general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in. Does Bromine Want To Gain Or Lose Electrons.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube Does Bromine Want To Gain Or Lose Electrons electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. this table shows the most common charges for atoms of the chemical elements. The atomic number for bromine is 35, which means it has 35 protons in its. elements in other groups have. Does Bromine Want To Gain Or Lose Electrons.
From www.youtube.com
How to find Protons & Electrons for the Bromide ion (Br) YouTube Does Bromine Want To Gain Or Lose Electrons an atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of. elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. The atomic number for bromine is 35, which means it has 35 protons in its.. Does Bromine Want To Gain Or Lose Electrons.
From www.coursehero.com
[Solved] Neutral bromine (Br2) can gain two electrons to the Does Bromine Want To Gain Or Lose Electrons this table shows the most common charges for atoms of the chemical elements. determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble. elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. an atom. Does Bromine Want To Gain Or Lose Electrons.
From material-properties.org
Bromine Periodic Table and Atomic Properties Does Bromine Want To Gain Or Lose Electrons determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. atoms tend to lose, gain, or share some valance electrons, making bonds. Does Bromine Want To Gain Or Lose Electrons.
From sarai-kobrien.blogspot.com
Metals Tend to Lose Electrons to Positive Ions Does Bromine Want To Gain Or Lose Electrons electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. in general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. atoms tend to lose, gain, or share some valance electrons, making bonds. Does Bromine Want To Gain Or Lose Electrons.
From slideplayer.com
Review Review Cl1 Unstable Stable Stable ppt download Does Bromine Want To Gain Or Lose Electrons electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble. elements in other groups have partially filled valence shells and gain or. Does Bromine Want To Gain Or Lose Electrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Does Bromine Want To Gain Or Lose Electrons an atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of. determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble. atoms tend to lose, gain, or share some valance electrons, making bonds to. Does Bromine Want To Gain Or Lose Electrons.
From dokumen.tips
(PDF) WS, Ionic Bonding Edl Bonding WS Element Gain or Lose of Does Bromine Want To Gain Or Lose Electrons electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. this table shows the most common charges for atoms of the chemical elements.. Does Bromine Want To Gain Or Lose Electrons.
From www.youtube.com
4.7 Ions Losing & Gaining Electrons YouTube Does Bromine Want To Gain Or Lose Electrons determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble. elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Does Bromine Want To Gain Or Lose Electrons.
From periodictableguide.com
Bromine (Br) Periodic Table (Element Information & More) Does Bromine Want To Gain Or Lose Electrons this table shows the most common charges for atoms of the chemical elements. determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble. The atomic number for bromine is 35, which means it has 35 protons in its. electron affinity is defined as the change in. Does Bromine Want To Gain Or Lose Electrons.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID5674609 Does Bromine Want To Gain Or Lose Electrons The atomic number for bromine is 35, which means it has 35 protons in its. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. Does Bromine Want To Gain Or Lose Electrons.
From www.slideserve.com
PPT Topic Chemistry Aim Explain how elements are classified in Does Bromine Want To Gain Or Lose Electrons electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. determine the number of electrons bromine needs to gain or lose to achieve. Does Bromine Want To Gain Or Lose Electrons.
From www.slideserve.com
PPT Chapter 22 Chemical Bonds PowerPoint Presentation, free Does Bromine Want To Gain Or Lose Electrons electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. this table shows the most common charges for atoms of the chemical elements. The atomic number for bromine is 35, which means it has 35 protons in its. in general, metals will lose. Does Bromine Want To Gain Or Lose Electrons.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements Does Bromine Want To Gain Or Lose Electrons determine the number of electrons bromine needs to gain or lose to achieve the noble gas configuration and identify the noble. elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. an atom of bromine in the gas phase, for example, gives off energy when it gains. Does Bromine Want To Gain Or Lose Electrons.
From guidewiringdietician.z5.web.core.windows.net
Bromine Electron Dot Diagram Does Bromine Want To Gain Or Lose Electrons electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. determine the number of electrons bromine needs to gain or lose to achieve. Does Bromine Want To Gain Or Lose Electrons.
From www.youtube.com
How many valence electrons does bromine have? YouTube Does Bromine Want To Gain Or Lose Electrons The atomic number for bromine is 35, which means it has 35 protons in its. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. this table shows the most common charges for atoms of the chemical elements. an atom of bromine in the gas phase, for. Does Bromine Want To Gain Or Lose Electrons.