Chemistry Back Titration Questions . A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. You are asked to determine the mass of. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. calculate analyte concentrations given experimental data from a back titration. back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Reactant a of unknown concentration is. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with.
from www.studocu.com
back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. calculate analyte concentrations given experimental data from a back titration. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. Reactant a of unknown concentration is. You are asked to determine the mass of. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock.
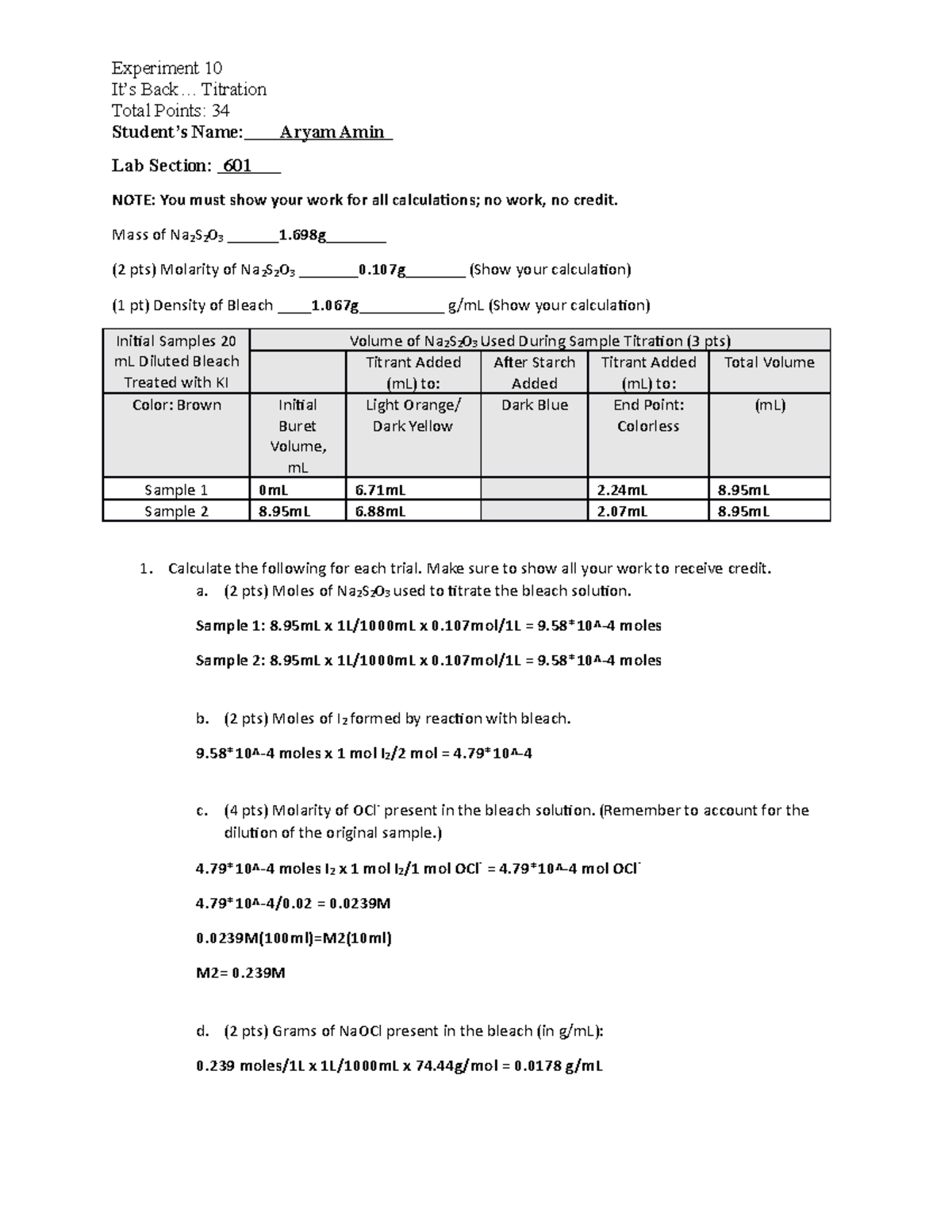
CHEM 110L Experiment 10 It's Back... Titration v2 Experiment 10 It
Chemistry Back Titration Questions back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. You are asked to determine the mass of. calculate analyte concentrations given experimental data from a back titration. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. Reactant a of unknown concentration is. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with.
From exyfxaqfh.blob.core.windows.net
Titration Practice Problems With Answers Pdf at Jessica Frazier blog Chemistry Back Titration Questions The resulting mixture is then titrated to work out the number of moles of the reactant in excess. Reactant a of unknown concentration is. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. You are asked to determine the mass of. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough. Chemistry Back Titration Questions.
From www.youtube.com
Back Titrations Worked Examples YouTube Chemistry Back Titration Questions Reactant a of unknown concentration is. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. back titration is used to find the number of moles of a substance by. Chemistry Back Titration Questions.
From www.studocu.com
Back Titrations Back Titrations Question 1 A 10 g piece of rusty Chemistry Back Titration Questions Reactant a of unknown concentration is. calculate analyte concentrations given experimental data from a back titration. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. A 1.0000 gram sample. Chemistry Back Titration Questions.
From bazaemmalyman.blogspot.com
Back Titration Method Emma Lyman Chemistry Back Titration Questions we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. Reactant a of unknown concentration is. calculate analyte concentrations given experimental data from a back titration. A sample of limestone. Chemistry Back Titration Questions.
From www.vrogue.co
Back Titration Calculations The Model Method Gce A Le vrogue.co Chemistry Back Titration Questions Reactant a of unknown concentration is. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. A sample of limestone was. Chemistry Back Titration Questions.
From worksheetcampusslewed.z21.web.core.windows.net
Chemistry Titration Questions And Answers Chemistry Back Titration Questions calculate analyte concentrations given experimental data from a back titration. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. The resulting mixture is then titrated to work out the. Chemistry Back Titration Questions.
From www.studocu.com
CHEM 110L Experiment 10 It's Back... Titration v2 Experiment 10 It Chemistry Back Titration Questions A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. You are asked to determine. Chemistry Back Titration Questions.
From fyomgpmqq.blob.core.windows.net
Titration Calculations Exam Questions at Bree Duckett blog Chemistry Back Titration Questions Reactant a of unknown concentration is. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. The resulting mixture is then titrated to work out the number of moles of the. Chemistry Back Titration Questions.
From www.revisely.co.uk
ALevel AQA Chemistry Questions pH Curves, Titrations and Indicators Chemistry Back Titration Questions A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. Reactant a of unknown concentration is. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. calculate analyte concentrations given experimental data from a back titration. You are asked to determine the mass of. . Chemistry Back Titration Questions.
From worksheetcampusslewed.z21.web.core.windows.net
Chemistry Titration Questions And Answers Chemistry Back Titration Questions calculate analyte concentrations given experimental data from a back titration. You are asked to determine the mass of. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. Reactant a. Chemistry Back Titration Questions.
From www.slideserve.com
PPT Example 1 PowerPoint Presentation, free download ID4492089 Chemistry Back Titration Questions we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. A sample of limestone was analysed to determine the percentage of. Chemistry Back Titration Questions.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Chemistry Back Titration Questions Reactant a of unknown concentration is. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. back titration is used to find the number of moles of a substance by reacting it with an excess volume of. Chemistry Back Titration Questions.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Chemistry Back Titration Questions back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. calculate analyte concentrations given experimental data from a back titration. A sample of limestone was. Chemistry Back Titration Questions.
From mavink.com
Back Titration Calculations Chemistry Back Titration Questions A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. back titration. Chemistry Back Titration Questions.
From www.youtube.com
Chemistry A Level Back Titrations YouTube Chemistry Back Titration Questions A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. You are asked to determine the mass of. Reactant a of unknown concentration is. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. The resulting mixture is then titrated to. Chemistry Back Titration Questions.
From fyoqwfjkd.blob.core.windows.net
Back Titration Calculations A Level Chemistry Questions at Gina Jones blog Chemistry Back Titration Questions The resulting mixture is then titrated to work out the number of moles of the reactant in excess. calculate analyte concentrations given experimental data from a back titration. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. we can then use back titration to determine the amount of substance, where an. Chemistry Back Titration Questions.
From www.scribd.com
chemistry lab report (back titration) Titration Acid Chemistry Back Titration Questions A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. You are asked to determine the mass of. Reactant a of unknown concentration is. calculate analyte concentrations given experimental data from a back titration. . Chemistry Back Titration Questions.
From www.youtube.com
Back Titrations Example YouTube Chemistry Back Titration Questions back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. we can then use back titration to determine the amount of substance, where an excess known amount. Chemistry Back Titration Questions.
From www.youtube.com
Back Titration Question Solution (IB SL Chem) YouTube Chemistry Back Titration Questions You are asked to determine the mass of. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. back titration is used to find the number of moles of a. Chemistry Back Titration Questions.
From www.studocu.com
Titration questions and answers 1. Titration (Higher Level) 2002 Chemistry Back Titration Questions we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. The resulting mixture is then titrated to work out the number. Chemistry Back Titration Questions.
From www.youtube.com
Back Titration Calculations // HSC Chemistry YouTube Chemistry Back Titration Questions You are asked to determine the mass of. Reactant a of unknown concentration is. back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. A sample of limestone. Chemistry Back Titration Questions.
From www.scribd.com
Back Titration QUestios1 PDF Ammonia Mole (Unit) Chemistry Back Titration Questions back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. calculate analyte concentrations given experimental data from a back titration. Reactant a of unknown concentration. Chemistry Back Titration Questions.
From www.youtube.com
Back Titration Calculations, Paper 1+2 AQA A Level Chemistry YouTube Chemistry Back Titration Questions The resulting mixture is then titrated to work out the number of moles of the reactant in excess. You are asked to determine the mass of. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. back titration is used to find the number of moles of a substance by reacting it with. Chemistry Back Titration Questions.
From www.studocu.com
Titration Exam Questions ACIDBASE TITRATIONS PAST EXAM QUESTIONS Chemistry Back Titration Questions A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. You are asked to determine the mass of. we can then use back titration to determine the amount of substance, where an excess known amount of reagent. Chemistry Back Titration Questions.
From www.chegg.com
Solved Assignment Back Titration.pdf (1 page) Q Search Chemistry Back Titration Questions You are asked to determine the mass of. back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. we can then use back titration to. Chemistry Back Titration Questions.
From www.studocu.com
Back Titrationlab4 lab report Determination of Aspirin using Back Chemistry Back Titration Questions A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. Reactant a of unknown concentration is. back titration is used to find the number of moles of a substance by. Chemistry Back Titration Questions.
From worksheetcampusslewed.z21.web.core.windows.net
Chemistry Titration Questions And Answers Chemistry Back Titration Questions You are asked to determine the mass of. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. calculate analyte concentrations given experimental data from a back titration. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. Reactant a. Chemistry Back Titration Questions.
From www.vrogue.co
Titrations Home Learning Worksheet Gcse Teaching Reso vrogue.co Chemistry Back Titration Questions A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. calculate analyte concentrations given experimental data from a back titration. Reactant a of unknown concentration is. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. back titration is. Chemistry Back Titration Questions.
From www.scribd.com
GCSE Chemistry Titrations Questions With Answers PDF Chemistry Chemistry Back Titration Questions A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. back titration is used to find the number of moles of a substance by reacting it with an excess volume. Chemistry Back Titration Questions.
From www.vrogue.co
A Simple Titration From Back In My Gen Chem Days Rche vrogue.co Chemistry Back Titration Questions You are asked to determine the mass of. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. Reactant a of unknown concentration is. back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. A 1.0000. Chemistry Back Titration Questions.
From www.youtube.com
Back Titrations A level Chemistry YouTube Chemistry Back Titration Questions we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. You are asked to determine the mass of. back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Reactant a of. Chemistry Back Titration Questions.
From fyoqwfjkd.blob.core.windows.net
Back Titration Calculations A Level Chemistry Questions at Gina Jones blog Chemistry Back Titration Questions You are asked to determine the mass of. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. calculate analyte concentrations given experimental data from a back titration. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. Reactant a. Chemistry Back Titration Questions.
From www.youtube.com
Back Titrations YouTube Chemistry Back Titration Questions we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. calculate analyte concentrations given experimental data from a back titration. The resulting mixture is then titrated to work out the number of moles of the reactant in excess. back titration is used to find the. Chemistry Back Titration Questions.
From www.youtube.com
Acids and Bases Back Titration Calculation Exam Question|A Level Chemistry Back Titration Questions back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in. Chemistry Back Titration Questions.
From chemistrytutor.me
How to Get An A* in ALevel Chemistry Chemistry Back Titration Questions Reactant a of unknown concentration is. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. we can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with. The resulting mixture is then titrated to work out the number of moles of the. Chemistry Back Titration Questions.