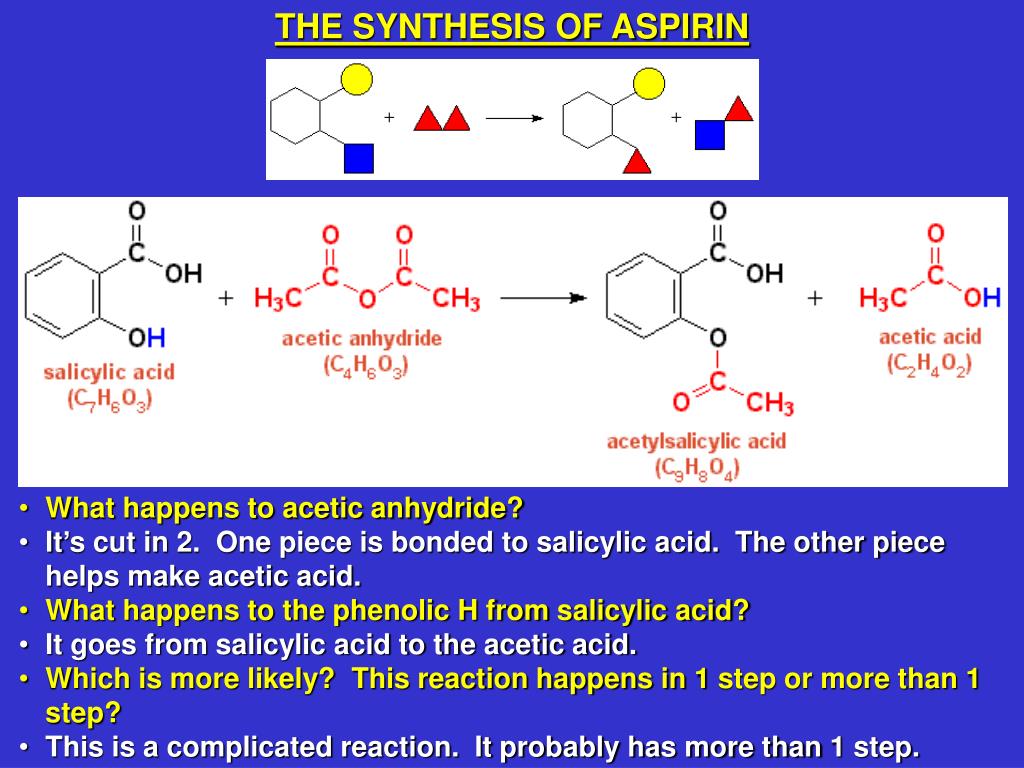
Equation Aspirin In Water . aspirin is slightly soluble in water: aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the temperature of human body, whereas the. The solubility of aspirin in water is 0.33 grams per 100 ml water at room. here is the equation for the reaction: Structures of aspirin and salicylic acid. The rate at which this reaction happens is. synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic anhydride gives. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. Salicylic acid and acetic acid anhydride react to form aspirin and.
from www.slideserve.com
The solubility of aspirin in water is 0.33 grams per 100 ml water at room. synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: here is the equation for the reaction: Structures of aspirin and salicylic acid. aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the temperature of human body, whereas the. The rate at which this reaction happens is. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. aspirin is slightly soluble in water: it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic anhydride gives. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used.
PPT CH 104 SYNTHESIS OF ASPIRIN AND OIL OF WINTERGREEN PowerPoint
Equation Aspirin In Water here is the equation for the reaction: here is the equation for the reaction: Salicylic acid and acetic acid anhydride react to form aspirin and. aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. The solubility of aspirin in water is 0.33 grams per 100 ml water at room. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. aspirin is slightly soluble in water: aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the temperature of human body, whereas the. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic anhydride gives. Structures of aspirin and salicylic acid. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: The rate at which this reaction happens is.
From www.youtube.com
Aspirin (C9H8O4) 3D Model with Lewis Structure YouTube Equation Aspirin In Water The rate at which this reaction happens is. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. Salicylic acid and acetic acid anhydride react to form aspirin and. aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. Structures of aspirin and salicylic acid. . Equation Aspirin In Water.
From www.coursehero.com
Solved What is the equation for the reaction that can Equation Aspirin In Water aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the temperature of human body, whereas the. Structures of aspirin and salicylic acid. here is the equation for the reaction: Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. The rate at which this reaction happens is.. Equation Aspirin In Water.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation ID6186176 Equation Aspirin In Water we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. here is the equation for the reaction: The solubility of aspirin in water is 0.33 grams per 100 ml water at room. synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: aspirin is dissolved in drinking water at. Equation Aspirin In Water.
From www.chegg.com
The solubility of aspirin in water is 1g per 300ml at Equation Aspirin In Water aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. aspirin is slightly soluble in water: we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. aspirin is dissolved in drinking water at ph 2 and 37 °c, which is. Equation Aspirin In Water.
From www.tessshebaylo.com
Balanced Chemical Equation For The Synthesis Of Aspirin Using Salicylic Equation Aspirin In Water aspirin is slightly soluble in water: Salicylic acid and acetic acid anhydride react to form aspirin and. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: aspirin is only slightly soluble in water and acidic solutions such as is present in the. Equation Aspirin In Water.
From www.slideserve.com
PPT CH 104 SYNTHESIS OF ASPIRIN AND OIL OF WINTERGREEN PowerPoint Equation Aspirin In Water The solubility of aspirin in water is 0.33 grams per 100 ml water at room. aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the temperature of human body, whereas the. Salicylic acid and acetic acid anhydride react to form aspirin and. Chemists sought to modify the salicylic acid molecule, reasoning that modification of. Equation Aspirin In Water.
From www.studocu.com
Lab 4 Synthesis of Aspirin Lab Partner Equation Aspirin In Water aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. Structures of aspirin and salicylic acid. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. here is the equation for the reaction: we can use equation \(\ref{eq1}\) to determine the reaction rate of. Equation Aspirin In Water.
From www.researchgate.net
Paracetamol and aspirin pathway. Side reactions solvation of aspirin Equation Aspirin In Water it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic anhydride gives. aspirin is slightly soluble in water: Salicylic acid and acetic acid anhydride react to form aspirin and. aspirin is only slightly soluble in water and acidic solutions such as is. Equation Aspirin In Water.
From chem.libretexts.org
Synthesis and Recrystallization of Aspirin Chemistry LibreTexts Equation Aspirin In Water synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. here is the equation for the reaction: Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. aspirin is slightly soluble in water:. Equation Aspirin In Water.
From www.numerade.com
SOLVED 6 In the synthesis of aspirin we react salicylic acid with Equation Aspirin In Water aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the temperature of human body, whereas the. synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: The rate at which this reaction happens is. The solubility of aspirin in water is 0.33 grams per 100 ml water at room. Salicylic acid and acetic acid anhydride. Equation Aspirin In Water.
From www.numerade.com
SOLVED Aspirin (acetylsalicylic acid HC9H7O4) is a weak acid. It Equation Aspirin In Water Salicylic acid and acetic acid anhydride react to form aspirin and. here is the equation for the reaction: Structures of aspirin and salicylic acid. aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: we can use equation \(\ref{eq1}\) to determine. Equation Aspirin In Water.
From www.nagwa.com
Question Video Identifying the Products from the Hydrolysis of Aspirin Equation Aspirin In Water Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. The rate at which this reaction happens is. The solubility of aspirin in water is 0.33 grams per 100 ml water at room. aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. we can. Equation Aspirin In Water.
From www.tessshebaylo.com
Chemical Equation Representing Synthesis Of Aspirin From Acetyl Equation Aspirin In Water it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic anhydride gives. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the. Equation Aspirin In Water.
From quizlet.com
What is the equation for the reaction that can Quizlet Equation Aspirin In Water The rate at which this reaction happens is. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. Salicylic acid and acetic acid anhydride react to form aspirin and. synthesis of. Equation Aspirin In Water.
From www.youtube.com
Aspirin Recrystallization Filtration YouTube Equation Aspirin In Water here is the equation for the reaction: it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic anhydride gives. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. The solubility of aspirin. Equation Aspirin In Water.
From www.coursehero.com
[Solved] The distribution coefficient of aspirin in ether/water at 25°C Equation Aspirin In Water synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: The rate at which this reaction happens is. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. aspirin is dissolved in drinking water at ph. Equation Aspirin In Water.
From www.scirp.org
Stability Studies of Lysine Acetylsalicylate (Aspirin Derivative Equation Aspirin In Water The rate at which this reaction happens is. here is the equation for the reaction: it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic anhydride gives. The solubility of aspirin in water is 0.33 grams per 100 ml water at room. Structures. Equation Aspirin In Water.
From www.youtube.com
Solubility of Aspirin (Explained) YouTube Equation Aspirin In Water aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. The solubility of aspirin in water is 0.33 grams per 100 ml water at room. aspirin is slightly soluble in water: Salicylic acid and acetic acid anhydride react to form aspirin and. here is the equation for the reaction: Structures. Equation Aspirin In Water.
From theory.labster.com
Synthesis of Aspirin Labster Equation Aspirin In Water Salicylic acid and acetic acid anhydride react to form aspirin and. aspirin is slightly soluble in water: Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. The rate at which this reaction happens is. aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the temperature of. Equation Aspirin In Water.
From studylib.net
Experiment AcidBase Titration of Aspirin Equation Aspirin In Water Structures of aspirin and salicylic acid. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. aspirin is slightly soluble in water: here is the equation for the reaction: Salicylic. Equation Aspirin In Water.
From www.numerade.com
SOLVED Write balanced reaction equations for the reactions involved (a Equation Aspirin In Water aspirin is slightly soluble in water: synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Salicylic acid and acetic acid anhydride react to form aspirin and. aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h. Equation Aspirin In Water.
From www.numerade.com
SOLVED Preparation of Aspirin Balance the equation for the synthesis Equation Aspirin In Water aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. Structures of aspirin and salicylic acid. aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the temperature of human body, whereas the. Salicylic acid and acetic acid anhydride react to form aspirin and. it uses. Equation Aspirin In Water.
From www.slideserve.com
PPT Aspirin Synthesis PowerPoint Presentation ID478355 Equation Aspirin In Water Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. Structures of aspirin and salicylic acid. aspirin is slightly soluble in water: Salicylic acid and acetic acid anhydride react to form aspirin and. synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: aspirin is dissolved in drinking water at ph 2 and. Equation Aspirin In Water.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID6186176 Equation Aspirin In Water it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic anhydride gives. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the. Equation Aspirin In Water.
From www.chegg.com
Solved Write an equation which shows why aspirin gives an Equation Aspirin In Water aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. The rate at which this reaction happens is. aspirin is slightly soluble in water: it uses a reaction of esterification catalyzed acid (h 2. Equation Aspirin In Water.
From byjus.com
Aspirin Formula Structural and Chemical Formula of Aspirin Equation Aspirin In Water aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the temperature of human body, whereas the. here is the equation for the reaction: synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. aspirin is only slightly. Equation Aspirin In Water.
From momentumclubs.org
😂 Aspirin synthesis reaction. What Is the Chemical Equation for the Equation Aspirin In Water The solubility of aspirin in water is 0.33 grams per 100 ml water at room. Salicylic acid and acetic acid anhydride react to form aspirin and. Structures of aspirin and salicylic acid. The rate at which this reaction happens is. synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: aspirin is slightly soluble in water: we can use. Equation Aspirin In Water.
From mungfali.com
Solved 2. Learning How Fast The Hydrolysis Of Aspirin Occurs 406 Equation Aspirin In Water Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. here is the equation for the reaction: The solubility of aspirin in water is 0.33 grams per 100 ml water at room. . Equation Aspirin In Water.
From www.saubhaya.com
Chemical Makeup Of Aspirin Saubhaya Makeup Equation Aspirin In Water it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic anhydride gives. aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the temperature of human body, whereas the. here is the equation for the reaction: we. Equation Aspirin In Water.
From ibalchemy.com
D.2 Aspirin and penicillin IB Alchemy Equation Aspirin In Water aspirin is slightly soluble in water: Structures of aspirin and salicylic acid. here is the equation for the reaction: The solubility of aspirin in water is 0.33 grams per 100 ml water at room. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. aspirin is dissolved in drinking water at. Equation Aspirin In Water.
From www.chegg.com
Solved water? 5. Aspirin gradually reacts with moisture in Equation Aspirin In Water it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic anhydride gives. The rate at which this reaction happens is. The solubility of aspirin in water is 0.33 grams per 100 ml water at room. aspirin is dissolved in drinking water at ph. Equation Aspirin In Water.
From www.slideserve.com
PPT Synthesis of Aspirin PowerPoint Presentation, free download ID Equation Aspirin In Water The rate at which this reaction happens is. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic anhydride gives. The solubility of aspirin in water is 0.33 grams per 100 ml water at room. Salicylic acid and acetic acid anhydride react to form. Equation Aspirin In Water.
From www.numerade.com
SOLVED Practice Exercise 3.34 Synthesis of Aspirin In the synthesis Equation Aspirin In Water Salicylic acid and acetic acid anhydride react to form aspirin and. aspirin is slightly soluble in water: Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: aspirin is dissolved in drinking water at ph 2 and 37 °c, which is the temperature. Equation Aspirin In Water.
From barmettler38394.blogspot.com
Synthesis Of Aspirin Without Acetic Anhydride Equation Aspirin In Water The rate at which this reaction happens is. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. aspirin is slightly soluble in water: aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. it uses a reaction of esterification catalyzed acid (h 2. Equation Aspirin In Water.
From fr.freepik.com
Vecteur de structure de formule chimique de la chimie de l'aspirine Equation Aspirin In Water Structures of aspirin and salicylic acid. Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. The rate at which this reaction happens is. here is the equation for the reaction: aspirin. Equation Aspirin In Water.