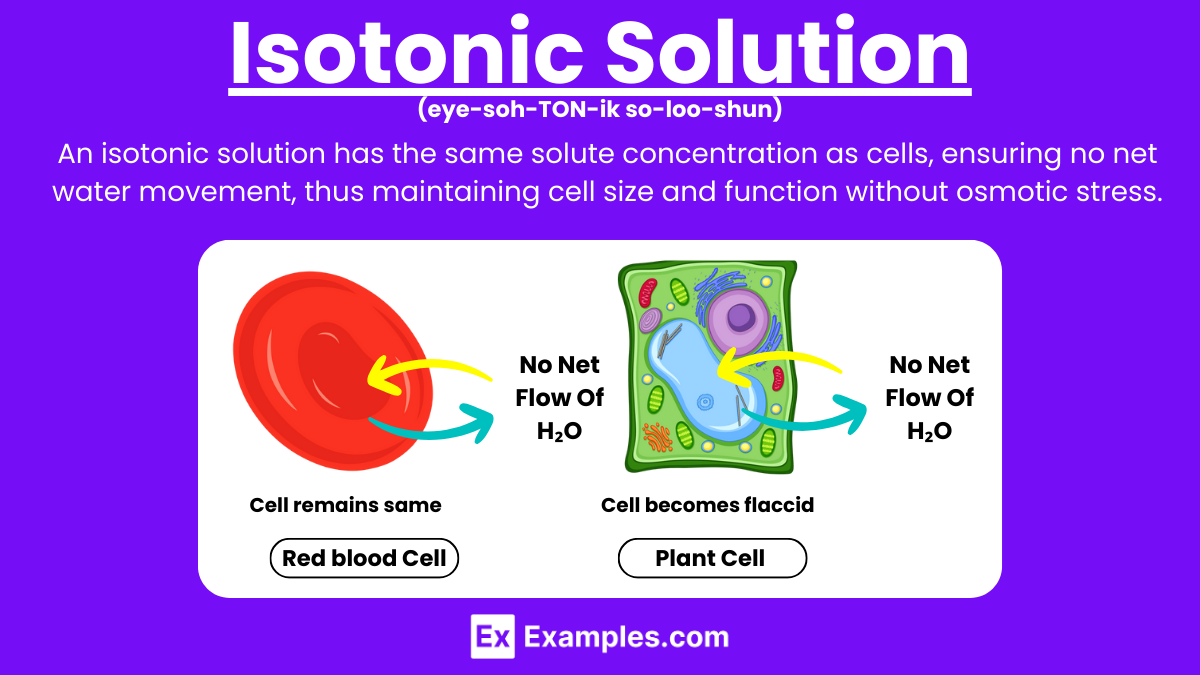
Example Of Isotonic Solution In Chemistry . an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. We use this term for describing solutions, chemistry, and muscles a few times in human biology. in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. If two solutions contain the same solute and water content, they are considered isotonic to each other (‘iso’ in isotonic means ‘same’ or ‘equal’). In chemistry, we call a. Apply osmosis to red blood cells and understand. what is an isotonic solution. It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water. differentiate and identify isotonic, hypertonic, and hypotonic solutions.
from www.examples.com
differentiate and identify isotonic, hypertonic, and hypotonic solutions. what is an isotonic solution. In chemistry, we call a. an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water. Apply osmosis to red blood cells and understand. in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. If two solutions contain the same solute and water content, they are considered isotonic to each other (‘iso’ in isotonic means ‘same’ or ‘equal’). solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. We use this term for describing solutions, chemistry, and muscles a few times in human biology. an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net.
Isotonic Solution Introduction, Examples, Uses, Difference
Example Of Isotonic Solution In Chemistry We use this term for describing solutions, chemistry, and muscles a few times in human biology. an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. We use this term for describing solutions, chemistry, and muscles a few times in human biology. in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. Apply osmosis to red blood cells and understand. differentiate and identify isotonic, hypertonic, and hypotonic solutions. an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water. In chemistry, we call a. It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. If two solutions contain the same solute and water content, they are considered isotonic to each other (‘iso’ in isotonic means ‘same’ or ‘equal’). what is an isotonic solution.
From www.slideserve.com
PPT The Cell Membrane PowerPoint Presentation, free download ID2276763 Example Of Isotonic Solution In Chemistry what is an isotonic solution. If two solutions contain the same solute and water content, they are considered isotonic to each other (‘iso’ in isotonic means ‘same’ or ‘equal’). Apply osmosis to red blood cells and understand. We use this term for describing solutions, chemistry, and muscles a few times in human biology. in chemistry, a solution is. Example Of Isotonic Solution In Chemistry.
From www.slideserve.com
PPT Osmosis PowerPoint Presentation, free download ID2180181 Example Of Isotonic Solution In Chemistry In chemistry, we call a. an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. If two solutions contain the same solute and water content, they are considered isotonic to each other (‘iso’ in isotonic means ‘same’ or ‘equal’). We use this term for describing solutions, chemistry, and muscles a. Example Of Isotonic Solution In Chemistry.
From study.com
Isotonic Solution Definition & Example Video & Lesson Transcript Example Of Isotonic Solution In Chemistry Apply osmosis to red blood cells and understand. an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. If two solutions contain the same solute and water content, they are considered isotonic. Example Of Isotonic Solution In Chemistry.
From www.eisonreports.com
Understanding Isotonic Solution Function, Uses, and Examples Example Of Isotonic Solution In Chemistry differentiate and identify isotonic, hypertonic, and hypotonic solutions. In chemistry, we call a. what is an isotonic solution. an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. solutions that contain the. Example Of Isotonic Solution In Chemistry.
From www.slideserve.com
PPT Remember! PowerPoint Presentation, free download ID2088564 Example Of Isotonic Solution In Chemistry It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. Apply osmosis to red blood cells and understand. In chemistry, we call a. solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. an isotonic solution is defined as two solutions of equal concentrations of solutes. Example Of Isotonic Solution In Chemistry.
From www.youtube.com
Hypertonic Isotonic Hypotonic Solution Simple Explanation of Example Of Isotonic Solution In Chemistry in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. We use this term for describing solutions, chemistry, and muscles a few times in human biology. an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. differentiate and identify. Example Of Isotonic Solution In Chemistry.
From pharmacyscope.com
Buffered Isotonic Solutions Pharmacy Scope Example Of Isotonic Solution In Chemistry an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water. It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. Apply osmosis to red blood cells and understand. what is an isotonic solution. solutions that contain the same concentration. Example Of Isotonic Solution In Chemistry.
From www.slideserve.com
PPT Cell Transport Review PowerPoint Presentation, free download ID Example Of Isotonic Solution In Chemistry We use this term for describing solutions, chemistry, and muscles a few times in human biology. in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water.. Example Of Isotonic Solution In Chemistry.
From elroyandriese03153.blogspot.com
What Are Some Examples Of Isotonic Hypertonic And Hypotonic Solutions Example Of Isotonic Solution In Chemistry We use this term for describing solutions, chemistry, and muscles a few times in human biology. solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. Apply osmosis to red blood cells and understand. In chemistry, we call a. what is an isotonic solution. differentiate and identify isotonic, hypertonic, and. Example Of Isotonic Solution In Chemistry.
From www.athleticinsight.com
Isotonic Solution Definition, How it Works, Examples, and Benefits Example Of Isotonic Solution In Chemistry differentiate and identify isotonic, hypertonic, and hypotonic solutions. In chemistry, we call a. We use this term for describing solutions, chemistry, and muscles a few times in human biology. solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. If two solutions contain the same solute and water content, they are. Example Of Isotonic Solution In Chemistry.
From ibiologia.com
Tonicity Hypotonic, Hyertonic & Isotonic Solutions Example Of Isotonic Solution In Chemistry what is an isotonic solution. In chemistry, we call a. an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water. Apply osmosis to red blood cells and understand. It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. We use. Example Of Isotonic Solution In Chemistry.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID Example Of Isotonic Solution In Chemistry in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water. Apply osmosis to red blood cells and understand. an isotonic solution is a solution with. Example Of Isotonic Solution In Chemistry.
From www.athleticinsight.com
Isotonic Solution Definition, How it Works, Examples, and Benefits Example Of Isotonic Solution In Chemistry It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. Apply osmosis to red blood cells and understand. If two solutions contain the same solute and water content, they are considered isotonic to each other (‘iso’ in. Example Of Isotonic Solution In Chemistry.
From www.scribd.com
Isotonic Solutions Chemistry Earth & Life Sciences Example Of Isotonic Solution In Chemistry an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. Apply osmosis to red blood cells and understand. If two solutions contain the same solute and water content, they are considered isotonic to each other. Example Of Isotonic Solution In Chemistry.
From www.studocu.com
Isotonic Solution Isotonic Solution Isotonic Solution is a solution Example Of Isotonic Solution In Chemistry In chemistry, we call a. an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. differentiate and identify isotonic, hypertonic, and hypotonic solutions. Apply osmosis to red blood cells and understand. what is. Example Of Isotonic Solution In Chemistry.
From www.examples.com
Isotonic Solution Introduction, Examples, Uses, Difference Example Of Isotonic Solution In Chemistry solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. We use this term for describing solutions, chemistry, and muscles a few times in human biology. in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. an isotonic solution is defined. Example Of Isotonic Solution In Chemistry.
From www.differencebetween.com
Difference Between Isotonic and Isosmotic Compare the Difference Example Of Isotonic Solution In Chemistry differentiate and identify isotonic, hypertonic, and hypotonic solutions. solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. Apply osmosis to red blood cells and understand. If two solutions contain the same solute and water content, they are considered isotonic to each other (‘iso’ in isotonic means ‘same’ or ‘equal’). . Example Of Isotonic Solution In Chemistry.
From www.slideserve.com
PPT Solution PowerPoint Presentation, free download ID3101864 Example Of Isotonic Solution In Chemistry solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. differentiate and identify isotonic, hypertonic, and hypotonic solutions. what is an isotonic solution. We use this term for describing solutions, chemistry, and muscles a few times in human biology. In chemistry, we call a. an isotonic solution is defined. Example Of Isotonic Solution In Chemistry.
From byjus.com
Isotonic solutions are the solutions having same Example Of Isotonic Solution In Chemistry It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. In chemistry, we call a. what is an isotonic solution. differentiate and identify isotonic, hypertonic, and hypotonic solutions. an isotonic solution is. Example Of Isotonic Solution In Chemistry.
From www.youtube.com
Isotonic & Buffer Solutions Part 02 Preparation of Isotonic Solutions Example Of Isotonic Solution In Chemistry differentiate and identify isotonic, hypertonic, and hypotonic solutions. what is an isotonic solution. Apply osmosis to red blood cells and understand. It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. We use this term. Example Of Isotonic Solution In Chemistry.
From www.slideshare.net
Isotonic solutions Example Of Isotonic Solution In Chemistry in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. Apply osmosis to red blood cells and understand. solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. We use this term for describing solutions, chemistry, and muscles a few times in. Example Of Isotonic Solution In Chemistry.
From www.vecteezy.com
Diagram showing isotonic solution 1235756 Vector Art at Vecteezy Example Of Isotonic Solution In Chemistry Apply osmosis to red blood cells and understand. an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water. differentiate and identify isotonic, hypertonic, and hypotonic. Example Of Isotonic Solution In Chemistry.
From www.biologyonline.com
Isotonic Definition and Examples Biology Online Dictionary Example Of Isotonic Solution In Chemistry differentiate and identify isotonic, hypertonic, and hypotonic solutions. We use this term for describing solutions, chemistry, and muscles a few times in human biology. If two solutions contain the same solute and water content, they are considered isotonic to each other (‘iso’ in isotonic means ‘same’ or ‘equal’). Apply osmosis to red blood cells and understand. an isotonic. Example Of Isotonic Solution In Chemistry.
From narodnatribuna.info
Isotonic Solution Definition Examples And Diagram Example Of Isotonic Solution In Chemistry differentiate and identify isotonic, hypertonic, and hypotonic solutions. an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water. It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. an isotonic solution is a solution with the same solute concentration. Example Of Isotonic Solution In Chemistry.
From www.sciencefacts.net
Isotonic Solution Definition, Meaning, Examples & Diagram Example Of Isotonic Solution In Chemistry an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water. If two solutions contain the same solute and water content, they are considered isotonic to each. Example Of Isotonic Solution In Chemistry.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID6402527 Example Of Isotonic Solution In Chemistry in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. Apply osmosis to red blood cells and understand. an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water. an isotonic solution is a solution with. Example Of Isotonic Solution In Chemistry.
From www.youtube.com
Types of solutionshypertonic hypotonic and isotonic explained YouTube Example Of Isotonic Solution In Chemistry In chemistry, we call a. in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. We use this term for describing solutions, chemistry, and muscles a few times in human. Example Of Isotonic Solution In Chemistry.
From www.slideserve.com
PPT Isotonic Solutions PowerPoint Presentation, free download ID Example Of Isotonic Solution In Chemistry solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. In chemistry, we call a. an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. If two. Example Of Isotonic Solution In Chemistry.
From brainly.in
define isotonic solution in chemistry Brainly.in Example Of Isotonic Solution In Chemistry in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. In chemistry, we call a. It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. what is an isotonic solution. We use this term for describing solutions, chemistry, and muscles a few times in. Example Of Isotonic Solution In Chemistry.
From www.slideshare.net
Chapter 3 Movement of Substances Lesson 2 Effects of isotonic, hypo… Example Of Isotonic Solution In Chemistry Apply osmosis to red blood cells and understand. We use this term for describing solutions, chemistry, and muscles a few times in human biology. solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. In chemistry, we call a. what is an isotonic solution. an isotonic solution is a solution. Example Of Isotonic Solution In Chemistry.
From www.slideserve.com
PPT Active Transport PowerPoint Presentation ID2510053 Example Of Isotonic Solution In Chemistry differentiate and identify isotonic, hypertonic, and hypotonic solutions. In chemistry, we call a. in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another. If two solutions contain the same solute and water content, they are considered isotonic to each other (‘iso’ in isotonic means ‘same’ or ‘equal’). Apply osmosis. Example Of Isotonic Solution In Chemistry.
From www.yourzdoctor.com
Isotonic Solutions and Applications of Isotonic Solutions Example Of Isotonic Solution In Chemistry an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water. differentiate and identify isotonic, hypertonic, and hypotonic solutions. In chemistry, we call a. an isotonic solution is a solution with the same solute concentration as the cell’s interior, resulting in no net. in. Example Of Isotonic Solution In Chemistry.
From www.slideshare.net
Chapter 3 Movement of Substances Lesson 2 Effects of isotonic, hypo… Example Of Isotonic Solution In Chemistry If two solutions contain the same solute and water content, they are considered isotonic to each other (‘iso’ in isotonic means ‘same’ or ‘equal’). solutions that contain the same concentration of water and solutes as the cell cytoplasm are called isotonic. In chemistry, we call a. differentiate and identify isotonic, hypertonic, and hypotonic solutions. We use this term. Example Of Isotonic Solution In Chemistry.
From www.pinterest.co.uk
Types of Solutions infographic diagram including isotonic hypertonic Example Of Isotonic Solution In Chemistry differentiate and identify isotonic, hypertonic, and hypotonic solutions. It is generally measured with respect to the cytoplasmic concentration or tonicity of the cell. If two solutions contain the same solute and water content, they are considered isotonic to each other (‘iso’ in isotonic means ‘same’ or ‘equal’). what is an isotonic solution. an isotonic solution is defined. Example Of Isotonic Solution In Chemistry.
From www.youtube.com
Hypertonic, Hypotonic and Isotonic Solutions! YouTube Example Of Isotonic Solution In Chemistry We use this term for describing solutions, chemistry, and muscles a few times in human biology. an isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water. in chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another.. Example Of Isotonic Solution In Chemistry.