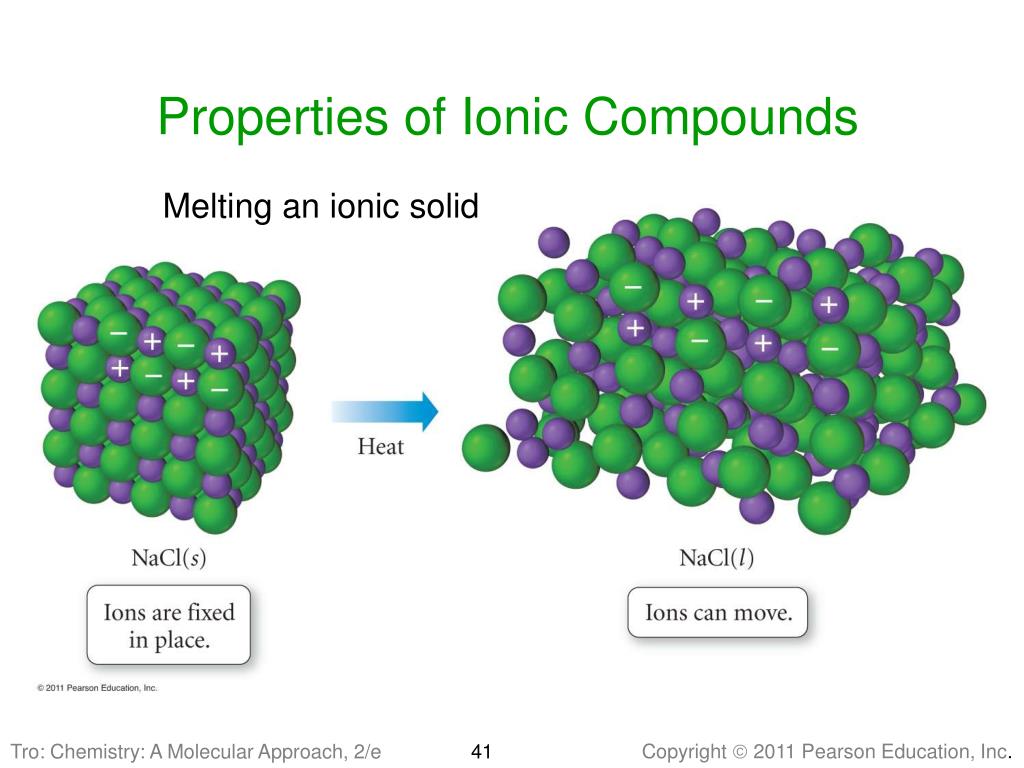
Lattice Structures Are 3D Models Of Ionic Compounds Structure . Each has its advantages and limitations. Most ionic, metallic and covalent compounds are crystalline lattice; Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. Different types of model are used to represent giant ionic structures. Ionic compounds are usually solids at room temperature, although ionic liquids also exist. The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. The ions, atoms or molecules are arranged in a regular and repeating arrangement; Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my.
from www.slideserve.com
Each has its advantages and limitations. Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge. The ions, atoms or molecules are arranged in a regular and repeating arrangement; Ionic compounds are usually solids at room temperature, although ionic liquids also exist. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. Most ionic, metallic and covalent compounds are crystalline lattice; Different types of model are used to represent giant ionic structures. Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions.
PPT Chapter 9 Chemical Bonding I Lewis Theory PowerPoint
Lattice Structures Are 3D Models Of Ionic Compounds Structure Most ionic, metallic and covalent compounds are crystalline lattice; The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge. Ionic compounds are usually solids at room temperature, although ionic liquids also exist. Different types of model are used to represent giant ionic structures. Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. The ions, atoms or molecules are arranged in a regular and repeating arrangement; 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. Most ionic, metallic and covalent compounds are crystalline lattice; Each has its advantages and limitations.
From saylordotorg.github.io
Chemical Compounds Lattice Structures Are 3D Models Of Ionic Compounds Structure Most ionic, metallic and covalent compounds are crystalline lattice; Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. Ionic compounds are usually solids at room temperature, although ionic liquids also exist. Different types of model are used to represent giant ionic structures. 3d drawings and models. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Lattice Structures Are 3D Models Of Ionic Compounds Structure Each has its advantages and limitations. Different types of model are used to represent giant ionic structures. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. The oppositely charged ions in a giant ionic lattice are held together by. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From keystagewiki.com
Giant Ionic Structure Key Stage Wiki Lattice Structures Are 3D Models Of Ionic Compounds Structure Ionic compounds are usually solids at room temperature, although ionic liquids also exist. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. Each has its advantages and limitations. Revision notes on ionic bonds & lattice structure for the cambridge. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From cartoondealer.com
Ionic Lattice Model Of Sodium And Chloride Molecules Cartoon Vector Lattice Structures Are 3D Models Of Ionic Compounds Structure The ions, atoms or molecules are arranged in a regular and repeating arrangement; Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. Each has its advantages and limitations. Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. Different types of model are. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From sciencenotes.org
Compounds With Both Ionic and Covalent Bonds Lattice Structures Are 3D Models Of Ionic Compounds Structure Most ionic, metallic and covalent compounds are crystalline lattice; Ionic compounds are usually solids at room temperature, although ionic liquids also exist. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. The oppositely charged ions in a giant ionic. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.vrogue.co
The Crystal Lattice Structure Of Ionic Compounds Info vrogue.co Lattice Structures Are 3D Models Of Ionic Compounds Structure Most ionic, metallic and covalent compounds are crystalline lattice; 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. The ions, atoms or molecules are arranged in a regular and repeating arrangement; Each has its advantages and limitations. Different types. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From bernieralice.hyperphp.com
Types Of Crystal Lattice Structure bernieralice Lattice Structures Are 3D Models Of Ionic Compounds Structure 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. Most ionic, metallic and covalent compounds are crystalline lattice; Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. Ionic compounds are usually solids at room temperature,. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.cgtrader.com
3D Printable Lattice Structures free 3D model 3D printable CGTrader Lattice Structures Are 3D Models Of Ionic Compounds Structure Each has its advantages and limitations. The ions, atoms or molecules are arranged in a regular and repeating arrangement; Most ionic, metallic and covalent compounds are crystalline lattice; The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge. Revision notes on ionic bonds & lattice structure for. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.cgtrader.com
3D Lattice Structures 3D model 3D printable CGTrader Lattice Structures Are 3D Models Of Ionic Compounds Structure Each has its advantages and limitations. Most ionic, metallic and covalent compounds are crystalline lattice; Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. The ions, atoms or molecules are arranged in a regular and repeating arrangement; Ionic compounds are usually solids at room temperature, although ionic liquids also exist. Different types of model are used. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From courses.lumenlearning.com
The Solid State of Matter Chemistry for Majors Lattice Structures Are 3D Models Of Ionic Compounds Structure Each has its advantages and limitations. Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. Revision notes on ionic bonds & lattice structure for the cambridge. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From 3dprint.com
3D Printed Aluminum Lattice Cube Weighs Just 3.9g & Holds up to 900lbs Lattice Structures Are 3D Models Of Ionic Compounds Structure Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. Ionic compounds are usually solids at room temperature, although ionic liquids also exist. The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge. 3d drawings and models depict the arrangement of ions in space,. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.researchgate.net
Commonly used lattice structures' geometry (ai) FCC unit cell and Lattice Structures Are 3D Models Of Ionic Compounds Structure Each has its advantages and limitations. The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge. Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. Most ionic, metallic and covalent compounds are crystalline lattice; Revision notes on ionic bonds & lattice structure for. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Lattice Structures Are 3D Models Of Ionic Compounds Structure Each has its advantages and limitations. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. The ions, atoms or molecules are arranged in a regular and repeating arrangement; Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.thesciencehive.co.uk
Properties of Compounds (AQA) — the science hive Lattice Structures Are 3D Models Of Ionic Compounds Structure Different types of model are used to represent giant ionic structures. Each has its advantages and limitations. Ionic compounds are usually solids at room temperature, although ionic liquids also exist. The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge. Structure 2.1.2—the ionic bond is formed by. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.esa.int
ESA Advanced Manufacturing Lattice Structures Are 3D Models Of Ionic Compounds Structure The ions, atoms or molecules are arranged in a regular and repeating arrangement; Each has its advantages and limitations. Ionic compounds are usually solids at room temperature, although ionic liquids also exist. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From chem.libretexts.org
Chapter 4.2 Lattice Energies in Ionic Solids Chemistry LibreTexts Lattice Structures Are 3D Models Of Ionic Compounds Structure Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. Different types of model are used to represent giant ionic structures. Most ionic, metallic and covalent compounds are crystalline lattice; Each has its advantages. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.cgtrader.com
3D Lattice Structures 3D model 3D printable CGTrader Lattice Structures Are 3D Models Of Ionic Compounds Structure The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge. Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. The ions, atoms or molecules are arranged in a regular and repeating arrangement; Each. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From zakruti.com
Lattice energy Molecular and ionic compound structure and properties AP Lattice Structures Are 3D Models Of Ionic Compounds Structure The ions, atoms or molecules are arranged in a regular and repeating arrangement; Ionic compounds are usually solids at room temperature, although ionic liquids also exist. Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. Each has its advantages and limitations. Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus,. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.tec-science.com
Important types of lattice structures tecscience Lattice Structures Are 3D Models Of Ionic Compounds Structure Different types of model are used to represent giant ionic structures. Ionic compounds are usually solids at room temperature, although ionic liquids also exist. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. The ions, atoms or molecules are. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From thechemistryclub.blogspot.com
The Chemistry Club Giant Ionic, Covalent, Giant Metallic and Simple Lattice Structures Are 3D Models Of Ionic Compounds Structure 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. Each has its advantages and limitations. Most ionic, metallic and covalent compounds are crystalline lattice; The oppositely charged ions in a giant ionic lattice are held together by strong ionic. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.linstitute.net
IB DP Chemistry SL复习笔记4.1.2 Ionic Compounds翰林国际教育 Lattice Structures Are 3D Models Of Ionic Compounds Structure Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge. Different types of model are used to represent giant ionic structures. The ions, atoms or molecules are arranged in a regular and repeating arrangement;. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From en.wikipedia.org
Ionic compound Wikipedia Lattice Structures Are 3D Models Of Ionic Compounds Structure Different types of model are used to represent giant ionic structures. Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. The ions, atoms or molecules are. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Lattice Structures Are 3D Models Of Ionic Compounds Structure Different types of model are used to represent giant ionic structures. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. Each has its advantages and limitations. The oppositely charged ions in a giant ionic lattice are held together by. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.graylark.com
Ionic Solids Lattice Structures Are 3D Models Of Ionic Compounds Structure The ions, atoms or molecules are arranged in a regular and repeating arrangement; Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. Different types of model are used to represent giant ionic structures. The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge.. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From cartoondealer.com
Ionic Compound Cubic Crystal Structure Cartoon Vector CartoonDealer Lattice Structures Are 3D Models Of Ionic Compounds Structure The ions, atoms or molecules are arranged in a regular and repeating arrangement; Most ionic, metallic and covalent compounds are crystalline lattice; The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge. Ionic compounds are usually solids at room temperature, although ionic liquids also exist. Each has. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.dreamstime.com
Ionic Compound, Crystal Structure with Positive and Negative Ions Lattice Structures Are 3D Models Of Ionic Compounds Structure Each has its advantages and limitations. The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge. Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. Different types of model are used to represent giant ionic structures. 3d drawings and models depict the arrangement. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I Lewis Theory PowerPoint Lattice Structures Are 3D Models Of Ionic Compounds Structure 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. Different types of model are used to. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.dreamstime.com
Ionic Lattice Process of Melting Stock Vector Illustration of binds Lattice Structures Are 3D Models Of Ionic Compounds Structure The ions, atoms or molecules are arranged in a regular and repeating arrangement; Most ionic, metallic and covalent compounds are crystalline lattice; Ionic compounds are usually solids at room temperature, although ionic liquids also exist. Each has its advantages and limitations. Different types of model are used to represent giant ionic structures. The oppositely charged ions in a giant ionic. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.9.1 Explaining Conductivity翰林国际教育 Lattice Structures Are 3D Models Of Ionic Compounds Structure The oppositely charged ions in a giant ionic lattice are held together by strong ionic bonds (electrostatic forces of attraction) in a huge. Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. Each has its advantages and limitations. Ionic compounds are usually solids at room temperature,. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.shutterstock.com
Vektor Stok Crystal Lattice Structure Ionic Compounds Ionic (Tanpa Lattice Structures Are 3D Models Of Ionic Compounds Structure The ions, atoms or molecules are arranged in a regular and repeating arrangement; Each has its advantages and limitations. Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. Different types of model are. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From brilliant.org
chemical bonding Brilliant Math & Science Wiki Lattice Structures Are 3D Models Of Ionic Compounds Structure The ions, atoms or molecules are arranged in a regular and repeating arrangement; Ionic compounds are usually solids at room temperature, although ionic liquids also exist. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d models only show the arrangement of. Each has its advantages. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.pinterest.com
The Crystal Lattice Structure of Ionic Compounds infographic diagram Lattice Structures Are 3D Models Of Ionic Compounds Structure Different types of model are used to represent giant ionic structures. The ions, atoms or molecules are arranged in a regular and repeating arrangement; Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. 3d drawings and models depict the arrangement of ions in space, showing the. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.slideserve.com
PPT Giant Ionic Structures PowerPoint Presentation, free download Lattice Structures Are 3D Models Of Ionic Compounds Structure Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. Different types of model are used to represent giant ionic structures. The ions, atoms or molecules are arranged in a regular and repeating arrangement; Most ionic, metallic and covalent compounds are crystalline lattice; Ionic compounds are usually. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.youtube.com
Lattice Structures in Ionic Solids YouTube Lattice Structures Are 3D Models Of Ionic Compounds Structure Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. Most ionic, metallic and covalent compounds are crystalline lattice; Different types of model are used to represent giant ionic structures. Structure 2.1.2—the ionic bond is formed by electrostatic attractions between oppositely charged ions. Ionic compounds are usually. Lattice Structures Are 3D Models Of Ionic Compounds Structure.
From www.youtube.com
Lattice Structures in 3DXpert YouTube Lattice Structures Are 3D Models Of Ionic Compounds Structure The ions, atoms or molecules are arranged in a regular and repeating arrangement; Revision notes on ionic bonds & lattice structure for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. 3d drawings and models depict the arrangement of ions in space, showing the repeating pattern of ions throughout giant ionic lattice structures (whereas 2d. Lattice Structures Are 3D Models Of Ionic Compounds Structure.