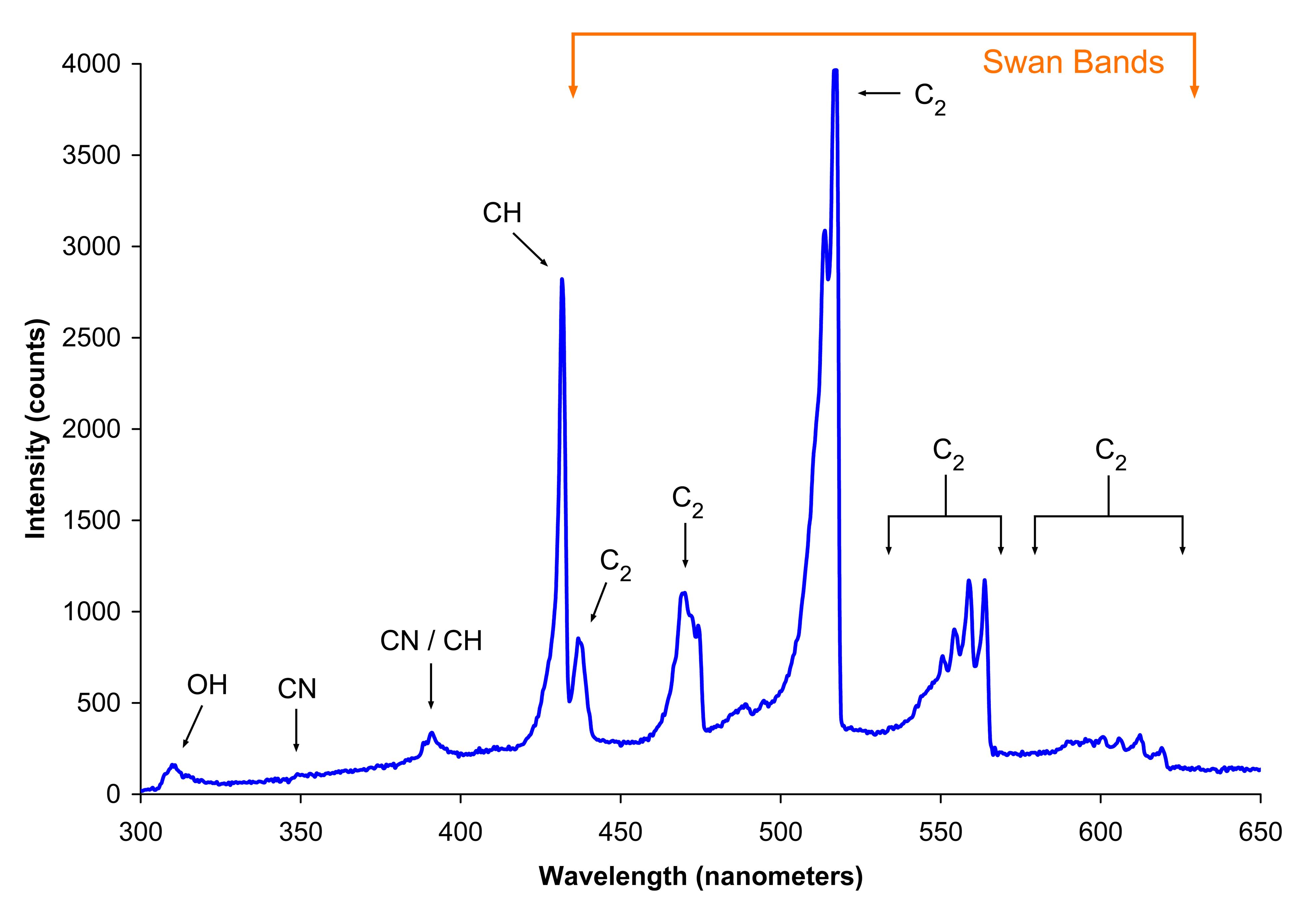
Spectra Flame Definition . Mostly the flame test detects. Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. During a flame test experiment, metal chlorides are directly placed into a flame. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Flame spectra the spectra of gaseous, atomic particles consist of well defined narrow discrete lines arising from electronic transition of. Not all metal ions give flame colors. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. The intense heat will promote the metal's electrons to an excited state. For group 1 compounds, flame tests are.
from www.wikidoc.org
Flame spectra the spectra of gaseous, atomic particles consist of well defined narrow discrete lines arising from electronic transition of. Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Not all metal ions give flame colors. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. The intense heat will promote the metal's electrons to an excited state. Mostly the flame test detects. During a flame test experiment, metal chlorides are directly placed into a flame. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. For group 1 compounds, flame tests are. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra.
Radical (chemistry) wikidoc
Spectra Flame Definition The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Flame spectra the spectra of gaseous, atomic particles consist of well defined narrow discrete lines arising from electronic transition of. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. Not all metal ions give flame colors. For group 1 compounds, flame tests are. Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Mostly the flame test detects. During a flame test experiment, metal chlorides are directly placed into a flame. The intense heat will promote the metal's electrons to an excited state. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra.
From www.studypool.com
SOLUTION Flame Emission Spectrophotometer? Its quality control Spectra Flame Definition For group 1 compounds, flame tests are. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. The intense heat will promote the metal's electrons to an excited state. Mostly the flame test detects. During a flame test experiment, metal chlorides are directly placed into a flame. Primarily, the flame test. Spectra Flame Definition.
From electricaltestmodules.blogspot.com
Electrical test modules Flame tests and emission spectra Spectra Flame Definition For group 1 compounds, flame tests are. Not all metal ions give flame colors. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. The intense heat will promote the metal's electrons to an excited state. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that. Spectra Flame Definition.
From studylib.net
Emission Spectra and Flame Tests Spectra Flame Definition Not all metal ions give flame colors. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. The flame test is an analytical chemistry technique that helps identify. Spectra Flame Definition.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Spectra Flame Definition The intense heat will promote the metal's electrons to an excited state. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. For group 1 compounds, flame tests are. During a flame test experiment, metal chlorides are directly placed into a flame. The flame test is an. Spectra Flame Definition.
From www.researchgate.net
Flame emission spectra measured from laminar methaneair flames at (a Spectra Flame Definition Mostly the flame test detects. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. During a flame test experiment, metal chlorides are directly placed into a flame. For group 1 compounds, flame tests are. Primarily, the flame test detects the presence of metal ions in a. Spectra Flame Definition.
From murphsblogofchemjoy.blogspot.com
Murph's Blog of Chem Joy SCH 4C Spectrum & Flame Tests Spectra Flame Definition For group 1 compounds, flame tests are. Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Not all metal ions give flame colors. During a flame test experiment, metal chlorides are directly placed into a flame. Flame photometry, also known as flame emission spectroscopy is a. Spectra Flame Definition.
From rkdmb.home.xs4all.nl
Flame spectroscopy Spectra Flame Definition Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. During a flame test experiment, metal chlorides are directly placed into a flame. Flame photometry, also known as. Spectra Flame Definition.
From www.researchgate.net
Flame emission spectra measured from laminar methaneair flames at (a Spectra Flame Definition The intense heat will promote the metal's electrons to an excited state. For group 1 compounds, flame tests are. Mostly the flame test detects. Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. During a flame test experiment, metal chlorides are directly placed into a flame.. Spectra Flame Definition.
From www.slideshare.net
Atomic Spectroscopy Basic Principles and Instruments Spectra Flame Definition Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Not all metal ions give flame colors. Flame spectra the spectra of gaseous, atomic particles consist of well. Spectra Flame Definition.
From pixels.com
Calcium Flame Test Photograph by Andrew Lambert Photography Pixels Spectra Flame Definition For group 1 compounds, flame tests are. Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Not all metal ions give flame colors. Flame photometry, also known. Spectra Flame Definition.
From www.youtube.com
Atomic Spectroscopy Flame Emission The Flame Test YouTube Spectra Flame Definition The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. For group 1 compounds, flame tests are. Flame spectra the spectra of gaseous, atomic particles consist of well defined narrow discrete lines arising from electronic transition of. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy. Spectra Flame Definition.
From www.studypool.com
SOLUTION Flame Emission Spectrophotometer? Its quality control Spectra Flame Definition The intense heat will promote the metal's electrons to an excited state. Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. The flame test is an analytical. Spectra Flame Definition.
From imagine.gsfc.nasa.gov
Spectra Introduction Spectra Flame Definition Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. During a flame test experiment, metal chlorides are directly placed into a flame. For. Spectra Flame Definition.
From www.pinterest.com
Pin on Technology Spectra Flame Definition The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Not all metal ions give flame colors. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. Mostly the flame test detects. The intense heat will promote. Spectra Flame Definition.
From www.chemtopper.com
Flame spectrum Spectra Flame Definition For group 1 compounds, flame tests are. During a flame test experiment, metal chlorides are directly placed into a flame. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Mostly. Spectra Flame Definition.
From nandeesukak.blogspot.com
Flame Atomic Absorption Spectroscopy Atomic absorption spectroscopy Spectra Flame Definition Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. The intense heat will promote the metal's electrons to an excited state. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Mostly the flame test detects.. Spectra Flame Definition.
From pediaa.com
Difference Between Absorption and Emission Spectra Definition Spectra Flame Definition Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Not all metal ions give flame colors. Flame spectra the spectra of gaseous, atomic particles consist of well. Spectra Flame Definition.
From studylib.net
FLAME TEST AND ATOMIC SPECTRA LAB Spectra Flame Definition Flame spectra the spectra of gaseous, atomic particles consist of well defined narrow discrete lines arising from electronic transition of. For group 1 compounds, flame tests are. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. During a flame test experiment, metal chlorides are directly placed into a flame. The. Spectra Flame Definition.
From sciencenotes.org
Visible Light Spectrum Wavelengths and Colors Spectra Flame Definition Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Flame spectra the spectra of gaseous, atomic particles consist of well defined narrow discrete lines arising from electronic transition of. Not all metal ions give flame colors. Mostly the flame test detects. For group 1 compounds, flame. Spectra Flame Definition.
From lucept.com
visible spectrum image Lucept Spectra Flame Definition During a flame test experiment, metal chlorides are directly placed into a flame. For group 1 compounds, flame tests are. Mostly the flame test detects. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned. Spectra Flame Definition.
From webmis.highland.cc.il.us
Atomic Spectra and Models of the Atom Spectra Flame Definition Mostly the flame test detects. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. The intense heat will promote the metal's electrons to an excited state. For. Spectra Flame Definition.
From mucholderthen.tumblr.com
Science Visualized • Flame color of various elements as compounds in... Spectra Flame Definition During a flame test experiment, metal chlorides are directly placed into a flame. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Mostly the flame test. Spectra Flame Definition.
From rkdmb.home.xs4all.nl
Flame spectroscopy Spectra Flame Definition Not all metal ions give flame colors. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. Flame spectra the spectra of gaseous, atomic particles consist of well. Spectra Flame Definition.
From mohamednewscaldwell.blogspot.com
Determination of Sodium by Flame Emission Spectroscopy Spectra Flame Definition Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. The intense heat will promote the metal's electrons to an excited state. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Flame tests are used to. Spectra Flame Definition.
From www.slideserve.com
PPT Flame Detector Types PowerPoint Presentation ID5749652 Spectra Flame Definition For group 1 compounds, flame tests are. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Mostly the flame test detects. Flame spectra the spectra of gaseous,. Spectra Flame Definition.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Spectra Flame Definition The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. During a flame test experiment, metal chlorides are directly placed into a flame. The intense heat will promote the metal's electrons to an excited state. Flame spectra the spectra of gaseous, atomic particles consist of well defined narrow discrete lines. Spectra Flame Definition.
From www.wikidoc.org
Radical (chemistry) wikidoc Spectra Flame Definition Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Not all metal ions give flame colors. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. For group 1 compounds, flame tests are. Flame photometry, also. Spectra Flame Definition.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Spectra Flame Definition Mostly the flame test detects. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. Not all metal ions give flame colors. The intense heat will promote the metal's electrons to an excited state. The flame test is an analytical chemistry technique that helps identify elements in. Spectra Flame Definition.
From www.azosensors.com
How UV, IR and Imaging Detectors Work Spectra Flame Definition Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. During a flame test experiment, metal chlorides are directly placed into a flame. Not all metal ions give flame colors. For group 1 compounds, flame tests are. Mostly the flame test detects. The flame test is an analytical chemistry technique that. Spectra Flame Definition.
From www.youtube.com
Flame photometry/Flame Emission Spectroscopy (FES)/Atomic emission Spectra Flame Definition Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. The intense heat will promote the metal's electrons to an excited state. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. During a flame test experiment, metal. Spectra Flame Definition.
From socratic.org
Why are atomic spectra of an element discontinuous? Socratic Spectra Flame Definition The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. The intense heat will promote the metal's electrons to an excited state. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. During a flame test experiment, metal chlorides are directly. Spectra Flame Definition.
From es.slideshare.net
Emission spectroscopy Spectra Flame Definition Mostly the flame test detects. During a flame test experiment, metal chlorides are directly placed into a flame. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. The intense heat will promote the metal's electrons to an excited state. The flame test is an analytical chemistry. Spectra Flame Definition.
From www.pinterest.com
Emission Spectrum Vs. Absorption Spectrum Astronomy lessons Spectra Flame Definition The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Primarily, the flame test detects the presence of metal ions in a compound, and as ions of each element have a specific characteristic. Flame tests are used to identify the presence of a relatively small number of metal ions in. Spectra Flame Definition.
From chemistry.com.pk
Metal Ion Flame Test Colours [Infographic] Spectra Flame Definition Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. During a flame test experiment, metal chlorides are directly placed into a flame. The intense heat will promote. Spectra Flame Definition.
From www.researchgate.net
Typical flame's spectrum (normalized) of natural gas, oil and biooil Spectra Flame Definition Flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in. During a flame test experiment, metal chlorides are directly placed into a flame. Not all metal ions give flame colors. Flame spectra the spectra of gaseous, atomic particles consist of well defined narrow discrete lines arising from. Spectra Flame Definition.