What Makes A Boiling Point Higher . Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher boiling. More carbons means a greater. The intermolecular forces increase with increasing polarization of bonds. As a rule, larger molecules have higher boiling and melting points. More carbons and hydrogens create a greater surface area. Higher melting and boiling points signify stronger noncovalent intermolecular forces. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. Consider the boiling points of increasingly larger hydrocarbons. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Consider the boiling points of increasingly larger hydrocarbons.
from www.chegg.com
The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. More carbons means a greater. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. Higher melting and boiling points signify stronger noncovalent intermolecular forces. More carbons and hydrogens create a greater surface area. Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher boiling. Consider the boiling points of increasingly larger hydrocarbons. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. The intermolecular forces increase with increasing polarization of bonds.
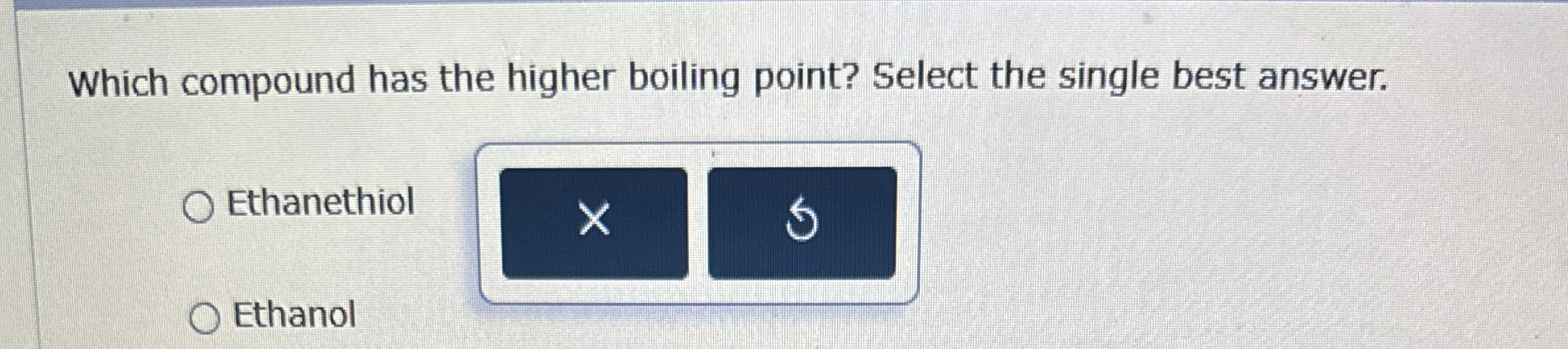
Solved Which compound has the higher boiling point? Select
What Makes A Boiling Point Higher The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Consider the boiling points of increasingly larger hydrocarbons. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. Consider the boiling points of increasingly larger hydrocarbons. Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher boiling. Higher melting and boiling points signify stronger noncovalent intermolecular forces. The intermolecular forces increase with increasing polarization of bonds. As a rule, larger molecules have higher boiling and melting points. More carbons and hydrogens create a greater surface area. More carbons means a greater. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Makes A Boiling Point Higher More carbons and hydrogens create a greater surface area. The intermolecular forces increase with increasing polarization of bonds. Higher melting and boiling points signify stronger noncovalent intermolecular forces. Consider the boiling points of increasingly larger hydrocarbons. More carbons means a greater. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. The boiling point of. What Makes A Boiling Point Higher.
From www.chegg.com
Solved Which compound has the higher boiling point? Select What Makes A Boiling Point Higher The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. The intermolecular forces increase with increasing polarization of bonds. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. More carbons means a greater. Large molecules have more electrons and. What Makes A Boiling Point Higher.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube What Makes A Boiling Point Higher Consider the boiling points of increasingly larger hydrocarbons. Consider the boiling points of increasingly larger hydrocarbons. Higher melting and boiling points signify stronger noncovalent intermolecular forces. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6. What Makes A Boiling Point Higher.
From scienceblogs.com
Water in Space What Happens? ScienceBlogs What Makes A Boiling Point Higher Higher melting and boiling points signify stronger noncovalent intermolecular forces. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. More carbons means a greater. The intermolecular forces increase with increasing polarization of bonds. More carbons and hydrogens. What Makes A Boiling Point Higher.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points Master Organic Chemistry What Makes A Boiling Point Higher Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher boiling. Consider the boiling points of increasingly larger hydrocarbons. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. The boiling point of a liquid depends on the intermolecular forces present between the. What Makes A Boiling Point Higher.
From haasl.weebly.com
How to find boiling point of a compound in hysys haasl What Makes A Boiling Point Higher Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher boiling. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you. What Makes A Boiling Point Higher.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point What Makes A Boiling Point Higher The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. The intermolecular forces increase with increasing. What Makes A Boiling Point Higher.
From www.youtube.com
Calculating boiling point elevation YouTube What Makes A Boiling Point Higher The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. More carbons and hydrogens create a greater surface area. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. The intermolecular forces increase with. What Makes A Boiling Point Higher.
From slideplayer.com
Intermolecular Forces ppt download What Makes A Boiling Point Higher Consider the boiling points of increasingly larger hydrocarbons. The intermolecular forces increase with increasing polarization of bonds. Consider the boiling points of increasingly larger hydrocarbons. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. The boiling point of. What Makes A Boiling Point Higher.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Makes A Boiling Point Higher Consider the boiling points of increasingly larger hydrocarbons. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. The intermolecular forces increase with increasing polarization of bonds. More carbons means a greater. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen.. What Makes A Boiling Point Higher.
From exovfosvo.blob.core.windows.net
What Is The Boiling Point Of Water On Venus at Gerald Fairchild blog What Makes A Boiling Point Higher The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. The intermolecular forces increase with increasing polarization of bonds. Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher boiling. Consider the boiling points of increasingly larger. What Makes A Boiling Point Higher.
From www.chegg.com
Solved Part DWhy is the boiling point of bromobenzene higher What Makes A Boiling Point Higher Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher boiling. Consider the boiling points of increasingly larger hydrocarbons. Consider the boiling points of increasingly larger hydrocarbons. As a rule, larger molecules have higher boiling and melting points. The strength of intermolecular forces (and therefore impact on boiling points) is. What Makes A Boiling Point Higher.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point What Makes A Boiling Point Higher The intermolecular forces increase with increasing polarization of bonds. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. Higher melting and boiling points signify stronger noncovalent intermolecular forces. More carbons and hydrogens create a greater surface area. The boiling point of a liquid depends on the. What Makes A Boiling Point Higher.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points — Master Organic Chemistry What Makes A Boiling Point Higher Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. Consider the boiling points of increasingly larger hydrocarbons. Consider the boiling points of increasingly larger hydrocarbons. The intermolecular forces increase with increasing polarization of bonds. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. Large molecules have. What Makes A Boiling Point Higher.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Makes A Boiling Point Higher The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. More carbons and hydrogens create a greater surface area. Consider the boiling points of increasingly larger hydrocarbons. The intermolecular forces increase with increasing polarization of bonds. More carbons means. What Makes A Boiling Point Higher.
From www.slideserve.com
PPT Melting Point and Boiling Point PowerPoint Presentation, free What Makes A Boiling Point Higher Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher boiling. More carbons and hydrogens create a greater surface area. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a. What Makes A Boiling Point Higher.
From gioxhprmf.blob.core.windows.net
Boiling Point Of Dot 4 Brake Fluid at Coreen Cabral blog What Makes A Boiling Point Higher The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. Consider the boiling points of increasingly larger hydrocarbons. More carbons and hydrogens create a greater surface area. The strength of. What Makes A Boiling Point Higher.
From jsmithmoore.com
Boiling point of ethanol celsius What Makes A Boiling Point Higher The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. More carbons and hydrogens create. What Makes A Boiling Point Higher.
From blog.thermoworks.com
Thermal Secrets to Boiling Point Calibration ThermoWorks What Makes A Boiling Point Higher The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Consider the boiling points of increasingly larger hydrocarbons. More carbons and hydrogens create a greater surface area. The strength of intermolecular forces (and therefore impact on boiling points) is. What Makes A Boiling Point Higher.
From www.youtube.com
Boiling Point from PVT Diagram (Example) YouTube What Makes A Boiling Point Higher Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. Consider the boiling points of increasingly larger hydrocarbons. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. The boiling point of a liquid depends on the intermolecular forces present. What Makes A Boiling Point Higher.
From www.nagwa.com
Question Video Identifying the Organic Molecule with the Highest What Makes A Boiling Point Higher The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. As a rule, larger molecules have higher boiling and melting points. The intermolecular forces increase with increasing polarization of bonds. More carbons and hydrogens create a greater surface area.. What Makes A Boiling Point Higher.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Alcohols What Makes A Boiling Point Higher As a rule, larger molecules have higher boiling and melting points. More carbons and hydrogens create a greater surface area. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must. What Makes A Boiling Point Higher.
From www.researchgate.net
Extraction at boiling point of solvents Download Scientific Diagram What Makes A Boiling Point Higher The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher boiling. Spherically shaped molecules generally have relatively high melting. What Makes A Boiling Point Higher.
From www.youtube.com
Higher boiling point practice General Chemistry 2 Chapter 12 YouTube What Makes A Boiling Point Higher Higher melting and boiling points signify stronger noncovalent intermolecular forces. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. More carbons and hydrogens create a greater surface area. As a rule, larger molecules have higher boiling and. What Makes A Boiling Point Higher.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Makes A Boiling Point Higher Higher melting and boiling points signify stronger noncovalent intermolecular forces. More carbons means a greater. Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher boiling. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt. What Makes A Boiling Point Higher.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Makes A Boiling Point Higher The intermolecular forces increase with increasing polarization of bonds. More carbons means a greater. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the. What Makes A Boiling Point Higher.
From www.youtube.com
Difference in Boiling Points for H2O and H2S YouTube What Makes A Boiling Point Higher The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. More carbons means a greater. Consider the boiling points of increasingly larger hydrocarbons. The intermolecular forces increase with increasing polarization of bonds. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the. What Makes A Boiling Point Higher.
From gioxhprmf.blob.core.windows.net
Boiling Point Of Dot 4 Brake Fluid at Coreen Cabral blog What Makes A Boiling Point Higher The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. More carbons and hydrogens create a greater surface area. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. Consider the boiling points of increasingly larger hydrocarbons. The intermolecular forces increase with increasing polarization of bonds. More carbons. What Makes A Boiling Point Higher.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Makes A Boiling Point Higher Higher melting and boiling points signify stronger noncovalent intermolecular forces. As a rule, larger molecules have higher boiling and melting points. More carbons means a greater. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. Large molecules have more electrons and nuclei that create van der. What Makes A Boiling Point Higher.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Makes A Boiling Point Higher Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher boiling. Spherically shaped molecules generally have relatively high melting points, which in some cases approach the boiling point. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you. What Makes A Boiling Point Higher.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country What Makes A Boiling Point Higher The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher boiling. As. What Makes A Boiling Point Higher.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Makes A Boiling Point Higher The intermolecular forces increase with increasing polarization of bonds. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Consider the boiling points of increasingly larger hydrocarbons. Large molecules have more electrons and nuclei that create van der waals. What Makes A Boiling Point Higher.
From diagramfricanofc.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point What Makes A Boiling Point Higher As a rule, larger molecules have higher boiling and melting points. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be. More carbons means a greater. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. Large molecules have more electrons and. What Makes A Boiling Point Higher.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Makes A Boiling Point Higher The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen. The intermolecular forces increase with increasing polarization of bonds. Consider the boiling points of increasingly larger hydrocarbons. Consider the boiling points of increasingly larger hydrocarbons. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees. What Makes A Boiling Point Higher.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Makes A Boiling Point Higher More carbons means a greater. Higher melting and boiling points signify stronger noncovalent intermolecular forces. Consider the boiling points of increasingly larger hydrocarbons. Consider the boiling points of increasingly larger hydrocarbons. More carbons and hydrogens create a greater surface area. Large molecules have more electrons and nuclei that create van der waals attractive forces, so their compounds usually have higher. What Makes A Boiling Point Higher.