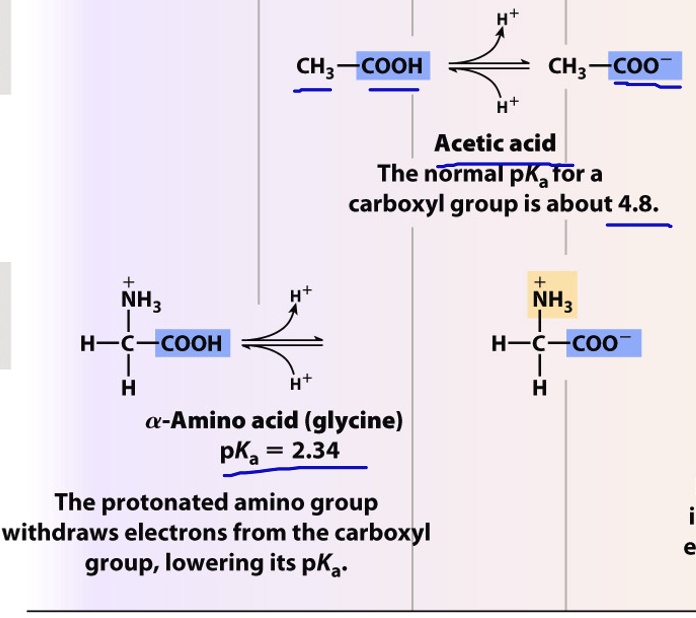
Amino Acids With Carboxyl Groups . It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino. all amino acids have the same basic structure, which is shown in figure 2.1. the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. At the “center” of each amino acid is a carbon.
from www.animalia-life.club
amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino. It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. At the “center” of each amino acid is a carbon. That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. all amino acids have the same basic structure, which is shown in figure 2.1.
Amino Group And Carboxyl Group
Amino Acids With Carboxyl Groups the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino. all amino acids have the same basic structure, which is shown in figure 2.1. At the “center” of each amino acid is a carbon.
From www.lecturio.com
Carboxylic Acids and their Derivatives Medical Library Amino Acids With Carboxyl Groups That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. the amine and carboxyl groups of an amino acid are both. Amino Acids With Carboxyl Groups.
From www.researchgate.net
A peptide bond is formed between two amino acid molecules when the Amino Acids With Carboxyl Groups the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. all amino acids have the same basic structure, which is shown in figure 2.1. amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group. Amino Acids With Carboxyl Groups.
From www.thoughtco.com
Amino Acids Structure, Classification and Function Amino Acids With Carboxyl Groups That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. At the “center” of each amino acid is a carbon. the. Amino Acids With Carboxyl Groups.
From www.researchgate.net
4. Two amino acids (the carboxylicgroup from the amino acid 1 and the Amino Acids With Carboxyl Groups It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. all amino acids have the same basic structure, which is shown in figure 2.1. At the “center” of each amino acid is a carbon. amino acids are linked to each other by peptide bonds, in which the. Amino Acids With Carboxyl Groups.
From www.coursehero.com
[Solved] Draw an amino acid and label the following amino group Amino Acids With Carboxyl Groups all amino acids have the same basic structure, which is shown in figure 2.1. the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. At the “center” of each. Amino Acids With Carboxyl Groups.
From www.slideserve.com
PPT Amino Acids and the Primary Structures of Proteins PowerPoint Amino Acids With Carboxyl Groups the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. At the “center” of each amino acid is a carbon. That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. amino acids are linked to each other by peptide bonds, in which the carboxyl group of. Amino Acids With Carboxyl Groups.
From byjus.com
Carboxylic Acids Properties, Nomenclature & Uses Chemistry Byju's Amino Acids With Carboxyl Groups It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. At the “center” of each amino acid is a carbon. all amino acids have the same basic structure, which is shown in figure 2.1. we saw in section 20.3 and section 24.5 that a carboxyl group is. Amino Acids With Carboxyl Groups.
From www.geeksforgeeks.org
Carboxylic Acids Structure, Examples, Properties, and Reactions Amino Acids With Carboxyl Groups At the “center” of each amino acid is a carbon. we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino. amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid. Amino Acids With Carboxyl Groups.
From bio.libretexts.org
S2019_Lecture_02_Reading Biology LibreTexts Amino Acids With Carboxyl Groups we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino. It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. the amine and carboxyl groups of an amino. Amino Acids With Carboxyl Groups.
From biology.stackexchange.com
biochemistry Difference in Basic Amino Structures Biology Stack Amino Acids With Carboxyl Groups all amino acids have the same basic structure, which is shown in figure 2.1. amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. the amine and carboxyl groups of an amino acid are. Amino Acids With Carboxyl Groups.
From www.priyamstudycentre.com
Amino Acids Definition, Formula, Structure, Types, Examples Amino Acids With Carboxyl Groups amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. At the “center” of each amino acid is a carbon. the amine and carboxyl groups of an amino acid are both covalently bonded to a. Amino Acids With Carboxyl Groups.
From courses.lumenlearning.com
Proteins OpenStax Biology 2e Amino Acids With Carboxyl Groups At the “center” of each amino acid is a carbon. the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. all amino acids have the same basic structure, which is shown in figure 2.1. It is this \(\ce{r}\) group which varies from one amino acid to another and is called. Amino Acids With Carboxyl Groups.
From www.animalia-life.club
Amino Group And Carboxyl Group Amino Acids With Carboxyl Groups That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. At the “center” of each amino acid is a carbon. It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. amino acids are linked to each other by peptide bonds, in which the carboxyl. Amino Acids With Carboxyl Groups.
From www.compoundchem.com
A Brief Guide to the Twenty Common Amino Acids Compound Interest Amino Acids With Carboxyl Groups amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. all amino acids have the same. Amino Acids With Carboxyl Groups.
From mavink.com
Amino Acid Structure Labeled Amino Acids With Carboxyl Groups It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. At the “center” of each amino acid is a carbon. amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. Amino Acids With Carboxyl Groups.
From www.slideserve.com
PPT Carboxylic Acids and Esters The functional group of carboxylic Amino Acids With Carboxyl Groups we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino. At the “center” of each amino acid is a carbon. That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. It is this \(\ce{r}\) group which. Amino Acids With Carboxyl Groups.
From www.animalia-life.club
Amino Group And Carboxyl Group Amino Acids With Carboxyl Groups It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph. Amino Acids With Carboxyl Groups.
From www.masterorganicchemistry.com
Simplifying the reactions of carboxylic acid derivatives (part 1 Amino Acids With Carboxyl Groups At the “center” of each amino acid is a carbon. amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and. Amino Acids With Carboxyl Groups.
From www.researchgate.net
(a) Components of an amino acid Ccentral carbon atom, Hhydrogen Amino Acids With Carboxyl Groups the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. At the “center” of each amino acid is a carbon. It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. That carbon atom is also bonded to a hydrogen atom. Amino Acids With Carboxyl Groups.
From www.dreamstime.com
Amino Acid Labeled Diagram Vector Illustration Drawing Biochemistry Amino Acids With Carboxyl Groups amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. It is this \(\ce{r}\) group which varies from one amino acid to. Amino Acids With Carboxyl Groups.
From www.lecturio.com
Basics of Amino Acids Concise Medical Knowledge Amino Acids With Carboxyl Groups we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino. all amino acids have the same basic structure, which is shown in figure 2.1. It is this \(\ce{r}\) group which varies from one amino acid to another and is. Amino Acids With Carboxyl Groups.
From chem.libretexts.org
12.1 Introduction to carboxylic acid derivatives and the nucleophilic Amino Acids With Carboxyl Groups It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. At the “center” of each amino acid is a carbon. amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. Amino Acids With Carboxyl Groups.
From www.chemistrysteps.com
Naming Carboxylic Acids Chemistry Steps Amino Acids With Carboxyl Groups all amino acids have the same basic structure, which is shown in figure 2.1. That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. amino acids are linked to each other by peptide bonds, in. Amino Acids With Carboxyl Groups.
From www.expii.com
Proteins — Overview & Importance in Biology Expii Amino Acids With Carboxyl Groups the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. At the “center” of each amino acid is a carbon. It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. That carbon atom is also bonded to a hydrogen atom. Amino Acids With Carboxyl Groups.
From courses.lumenlearning.com
Carboxylic Acids Introduction to Chemistry Amino Acids With Carboxyl Groups amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. we saw in section. Amino Acids With Carboxyl Groups.
From microbenotes.com
Amino Acids Properties, Structure, Classification, Functions Amino Acids With Carboxyl Groups amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. we saw in section. Amino Acids With Carboxyl Groups.
From www.pinterest.com
amino acids with pKas of most Rgroups AND backbone groups Biology Amino Acids With Carboxyl Groups At the “center” of each amino acid is a carbon. It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. all amino acids have the same basic structure, which is shown in figure 2.1. That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group.. Amino Acids With Carboxyl Groups.
From ar.inspiredpencil.com
Carboxylic Acid Group Amino Acids With Carboxyl Groups the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate. Amino Acids With Carboxyl Groups.
From www.chemistrystudent.com
Amino Acids and Proteins (ALevel) ChemistryStudent Amino Acids With Carboxyl Groups amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next, with the loss of a. At the “center” of each amino acid is a carbon. the amine and carboxyl groups of an amino acid are both covalently bonded to a. Amino Acids With Carboxyl Groups.
From chem.libretexts.org
1.2 Functional groups and organic nomenclature Chemistry LibreTexts Amino Acids With Carboxyl Groups It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. all amino acids have the same basic structure, which is shown in figure 2.1. amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the. Amino Acids With Carboxyl Groups.
From www.animalia-life.club
Amino Group And Carboxyl Group Amino Acids With Carboxyl Groups It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph. Amino Acids With Carboxyl Groups.
From psychology.wikia.com
Carboxylic acid Psychology Wiki Amino Acids With Carboxyl Groups all amino acids have the same basic structure, which is shown in figure 2.1. we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino. At the “center” of each amino acid is a carbon. That carbon atom is also. Amino Acids With Carboxyl Groups.
From www.sydney.edu.au
CARBOXYLIC ACIDS Amino Acids With Carboxyl Groups the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. At the “center” of each amino acid is a carbon. It is this \(\ce{r}\) group which varies from one amino acid to another and is called the amino acid side chain. all amino acids have the same basic structure, which. Amino Acids With Carboxyl Groups.
From studylib.net
Carboxylic Acids and Esters Amino Acids With Carboxyl Groups At the “center” of each amino acid is a carbon. That carbon atom is also bonded to a hydrogen atom and an \(\ce{r}\) group. we saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino. the amine and carboxyl groups. Amino Acids With Carboxyl Groups.
From www.researchgate.net
Amino acids and peptide bonds (A) Amino acids consist of a carbon atom Amino Acids With Carboxyl Groups all amino acids have the same basic structure, which is shown in figure 2.1. the amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. At the “center” of each amino acid is a carbon. It is this \(\ce{r}\) group which varies from one amino acid to another and is called. Amino Acids With Carboxyl Groups.