In A Titration Experiment H2O2 . Determine the concentration of the hydrogen peroxide solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. what you need to know. conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: To determine the concentration of hydrogen peroxide in solution, the method called manganometry. Measure the potential change of the reaction.
from dxopjnnhs.blob.core.windows.net
Determine the concentration of the hydrogen peroxide solution. Measure the potential change of the reaction. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: what you need to know.
A Level Chemistry Core Practical 11 Redox Titration at Abby Moseley blog
In A Titration Experiment H2O2 conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. Determine the concentration of the hydrogen peroxide solution. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Measure the potential change of the reaction. conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: what you need to know.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple In A Titration Experiment H2O2 Determine the concentration of the hydrogen peroxide solution. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. what you need to know. conduct the potentiometric. In A Titration Experiment H2O2.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC In A Titration Experiment H2O2 Measure the potential change of the reaction. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. what you need to know. a titration is a volumetric. In A Titration Experiment H2O2.
From exofzxxur.blob.core.windows.net
Hydrogen Peroxide Titration Assay at James Overcash blog In A Titration Experiment H2O2 a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. what you need to know. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \:. In A Titration Experiment H2O2.
From exoopidik.blob.core.windows.net
Titration Of H2O2 at Nicholas Walker blog In A Titration Experiment H2O2 Determine the concentration of the hydrogen peroxide solution. conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: what you need to know. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. a titration is a. In A Titration Experiment H2O2.
From mungfali.com
Titration Steps In A Titration Experiment H2O2 in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: To determine the concentration of hydrogen peroxide in solution, the method called manganometry. conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. In A Titration Experiment H2O2.
From www.sliderbase.com
Titration In A Titration Experiment H2O2 To determine the concentration of hydrogen peroxide in solution, the method called manganometry. Determine the concentration of the hydrogen peroxide solution. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte). In A Titration Experiment H2O2.
From www.vecteezy.com
Acid base titration experiment and phases of color change during In A Titration Experiment H2O2 in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: To determine the concentration of hydrogen peroxide in solution, the method called manganometry. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. conduct the potentiometric titration of. In A Titration Experiment H2O2.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages In A Titration Experiment H2O2 a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: To determine the concentration of hydrogen peroxide in solution, the method called manganometry. conduct the potentiometric titration of. In A Titration Experiment H2O2.
From www.chegg.com
Solved Data Table 1 Titration of 1.00 mL H2O2 with KMnO4 In A Titration Experiment H2O2 what you need to know. conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Measure the potential change of the reaction. Determine the concentration of the hydrogen. In A Titration Experiment H2O2.
From www.youtube.com
Titration MnO4 + H2O2 YouTube In A Titration Experiment H2O2 Determine the concentration of the hydrogen peroxide solution. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. a titration is a volumetric technique in which a solution. In A Titration Experiment H2O2.
From www.youtube.com
In a titration experiment, H2O2(aq) reacts with aqueous MnO4^(aq) as In A Titration Experiment H2O2 Measure the potential change of the reaction. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: To determine the concentration of hydrogen peroxide in solution, the method called. In A Titration Experiment H2O2.
From www.youtube.com
️ Titration Experiment for Board Class Complete Video to Understand In A Titration Experiment H2O2 To determine the concentration of hydrogen peroxide in solution, the method called manganometry. what you need to know. Determine the concentration of the hydrogen peroxide solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Measure the potential change. In A Titration Experiment H2O2.
From www.reddit.com
Struggling AP student here using a titration with potassium In A Titration Experiment H2O2 Measure the potential change of the reaction. what you need to know. Determine the concentration of the hydrogen peroxide solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. in a titration of sulfuric acid against sodium hydroxide,. In A Titration Experiment H2O2.
From www.studocu.com
YAB 2052Experiment 1 titration of hydrogen peroxide Applied In A Titration Experiment H2O2 To determine the concentration of hydrogen peroxide in solution, the method called manganometry. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Determine the concentration of the hydrogen peroxide solution. in a titration of sulfuric acid against sodium hydroxide,. In A Titration Experiment H2O2.
From www.pinterest.com
Titration experiment Applied science, Experiments, How to apply In A Titration Experiment H2O2 conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Measure the potential change of the reaction. what you need to know. a titration is a volumetric. In A Titration Experiment H2O2.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali In A Titration Experiment H2O2 what you need to know. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. a titration is a volumetric technique in which a solution of one. In A Titration Experiment H2O2.
From www.numerade.com
In a titration experiment, H2O2(aq) reacts with aqueous MnO4^(aq) as In A Titration Experiment H2O2 Determine the concentration of the hydrogen peroxide solution. Measure the potential change of the reaction. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: To determine the concentration. In A Titration Experiment H2O2.
From dxopjnnhs.blob.core.windows.net
A Level Chemistry Core Practical 11 Redox Titration at Abby Moseley blog In A Titration Experiment H2O2 conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. Determine the concentration of the hydrogen peroxide solution. what you need to know. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. . In A Titration Experiment H2O2.
From www.ingridscience.ca
Hydrogen peroxide chemistry ingridscience.ca In A Titration Experiment H2O2 in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: To determine the concentration of hydrogen peroxide in solution, the method called manganometry. conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. Determine the concentration of the hydrogen peroxide solution. Measure the potential change of the reaction. a titration is. In A Titration Experiment H2O2.
From www.researchgate.net
Comparison of released H2 amounts from titration experiments where Si In A Titration Experiment H2O2 in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Measure the potential change of the reaction. what you need to know. Determine the concentration of the hydrogen. In A Titration Experiment H2O2.
From theedge.com.hk
Chemistry How To Titration The Edge In A Titration Experiment H2O2 a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Determine the concentration of the hydrogen peroxide solution. To determine the concentration of hydrogen peroxide in solution, the method. In A Titration Experiment H2O2.
From dxoaafwnm.blob.core.windows.net
Redox Titration Experiment Procedure at Margaret Campos blog In A Titration Experiment H2O2 Determine the concentration of the hydrogen peroxide solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Measure the potential change of the reaction. conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate.. In A Titration Experiment H2O2.
From www.toppr.com
0.2 g of a sample of H2O2 required 10 mL of 1 N KMnO4 in a titration in In A Titration Experiment H2O2 To determine the concentration of hydrogen peroxide in solution, the method called manganometry. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Determine the concentration of the hydrogen peroxide solution. Measure the potential change of the reaction. in a. In A Titration Experiment H2O2.
From www.vernier.com
Potentiometric Titration of Hydrogen Peroxide > Experiment 32 from In A Titration Experiment H2O2 conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Measure the potential change of the reaction. Determine the concentration of the hydrogen peroxide solution.. In A Titration Experiment H2O2.
From www.numerade.com
SOLVED The reaction between MnO4 (aq) and H2O2 (aq) in acidic solution In A Titration Experiment H2O2 a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: To determine the concentration of hydrogen peroxide in solution, the method called manganometry. Determine the concentration of the hydrogen. In A Titration Experiment H2O2.
From www.vecteezy.com
Acid base titration experiment and phases of color change during In A Titration Experiment H2O2 Measure the potential change of the reaction. what you need to know. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. Determine the. In A Titration Experiment H2O2.
From www.youtube.com
Catalysts + H2O2 demonstration EXPERIMENT YouTube In A Titration Experiment H2O2 To determine the concentration of hydrogen peroxide in solution, the method called manganometry. what you need to know. Measure the potential change of the reaction. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. a titration is a volumetric. In A Titration Experiment H2O2.
From studylib.net
Formal Lab 2 H2O2 Redox Titration In A Titration Experiment H2O2 Measure the potential change of the reaction. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. what you need to know. Determine the concentration of the hydrogen peroxide solution. To determine the concentration of hydrogen peroxide in solution, the. In A Titration Experiment H2O2.
From www.researchgate.net
Reactivity of PM NPs toward H2O2 (a) Reaction MnO2 with H2O2 to In A Titration Experiment H2O2 To determine the concentration of hydrogen peroxide in solution, the method called manganometry. Measure the potential change of the reaction. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. conduct the potentiometric titration of the reaction between commercially available. In A Titration Experiment H2O2.
From www.youtube.com
of of Hydrogen Peroxide Chemical In A Titration Experiment H2O2 To determine the concentration of hydrogen peroxide in solution, the method called manganometry. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. In A Titration Experiment H2O2.
From www.researchgate.net
of a H2O2 and b hydroxylamine in the specific experiment In A Titration Experiment H2O2 Determine the concentration of the hydrogen peroxide solution. conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. Measure the potential change of the reaction. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. what you need to know. a titration is a volumetric technique in which a. In A Titration Experiment H2O2.
From dxompsokn.blob.core.windows.net
Lab Manual Titration at Charles Fisher blog In A Titration Experiment H2O2 Measure the potential change of the reaction. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. Determine the concentration of the hydrogen peroxide solution. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. a titration is. In A Titration Experiment H2O2.
From studylib.net
The Potentiometric Titration of Hydrogen Peroxide In A Titration Experiment H2O2 Measure the potential change of the reaction. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. what you need to know. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: To determine the concentration of hydrogen. In A Titration Experiment H2O2.
From studylib.net
14 Titration of H2O2 In A Titration Experiment H2O2 To determine the concentration of hydrogen peroxide in solution, the method called manganometry. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Determine the concentration of the hydrogen peroxide solution. conduct the potentiometric titration of the reaction between commercially. In A Titration Experiment H2O2.
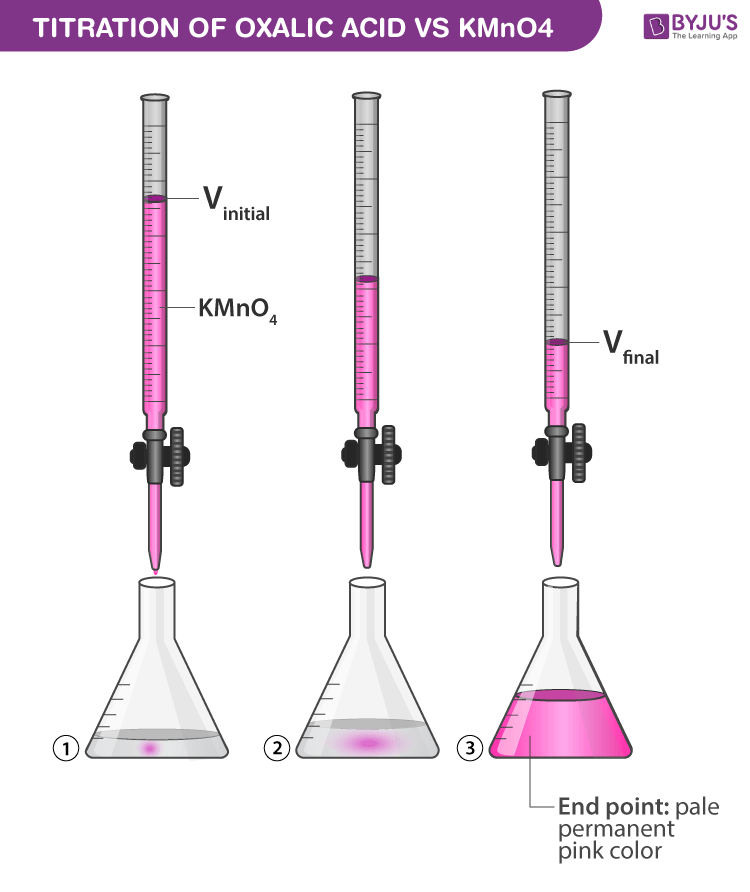
From byjus.com
0.2 g sample of H2O2 required 10 ml of 1N KMnO4 for complete reaction In A Titration Experiment H2O2 conduct the potentiometric titration of the reaction between commercially available hydrogen peroxide and potassium permanganate. Measure the potential change of the reaction. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. in a titration of sulfuric acid against. In A Titration Experiment H2O2.