Nitrogen Oxide React With Water . Alkali metals react with oxygen to form monoxides, peroxides, or superoxides. So 3 reacts with water as follows: Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion. No 2 (aq) + h 2 o (l) + 1½o 2 (g) → 2hno 3 (aq) when the clouds. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble in water (therefore, nitric acid exists as an. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid in acid rain. Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. Oxides of group 1 elements also react with water to create basic solutions.
from www.chegg.com
The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid in acid rain. So 3 reacts with water as follows: Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble in water (therefore, nitric acid exists as an. No 2 (aq) + h 2 o (l) + 1½o 2 (g) → 2hno 3 (aq) when the clouds. 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Oxides of group 1 elements also react with water to create basic solutions. Alkali metals react with oxygen to form monoxides, peroxides, or superoxides. Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion.
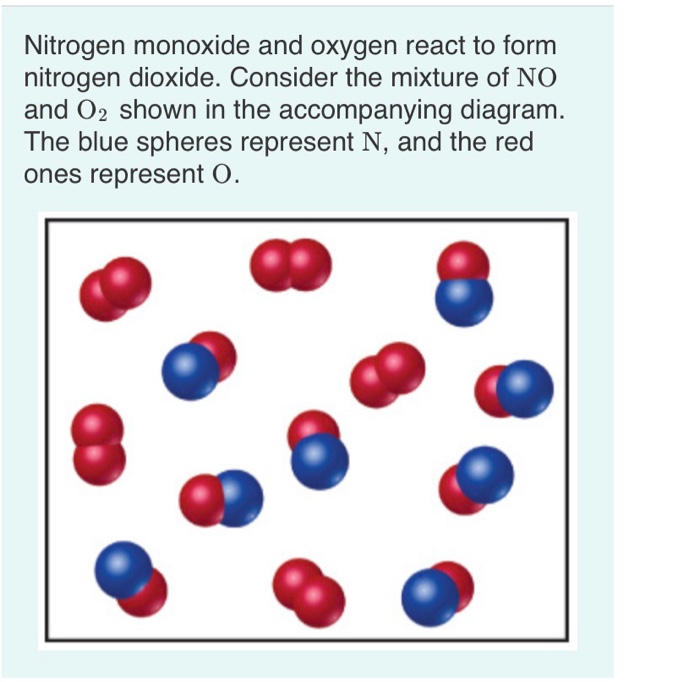
Solved Nitrogen monoxide and oxygen react to form nitrogen
Nitrogen Oxide React With Water 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid in acid rain. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble in water (therefore, nitric acid exists as an. Alkali metals react with oxygen to form monoxides, peroxides, or superoxides. No 2 (aq) + h 2 o (l) + 1½o 2 (g) → 2hno 3 (aq) when the clouds. Oxides of group 1 elements also react with water to create basic solutions. Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. So 3 reacts with water as follows: 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows:
From www.teachoo.com
[MCQ] Which of the statements is not correct? All metal oxides react Nitrogen Oxide React With Water The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: So 3 reacts with water as follows: 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow. Nitrogen Oxide React With Water.
From www.studyxapp.com
nitrogen monoxide and hydrogen react to form nitrogen and water like Nitrogen Oxide React With Water The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: No 2 (aq) + h 2 o (l) + 1½o 2 (g) → 2hno 3 (aq) when the clouds. 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. Although nitric oxide is thermodynamically. Nitrogen Oxide React With Water.
From www.numerade.com
Metal oxides can react with water to form bases. Nitrogen Oxide React With Water Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. No 2 (aq) + h 2 o (l) + 1½o 2 (g) → 2hno 3 (aq) when the clouds. Oxides of group 1 elements also react with water to create basic solutions. Gaseous. Nitrogen Oxide React With Water.
From www.chegg.com
Solved Nitric acid and nitrogen monoxide react to form Nitrogen Oxide React With Water The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Nitrogen dioxide in the air. Nitrogen Oxide React With Water.
From www.youtube.com
Zinc metal reaction Nitric acid reaction Zinc in nitric acid Nitrogen Oxide React With Water The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Oxides of group 1 elements also react with water to create basic solutions. No 2 (aq) + h 2 o (l) + 1½o 2 (g) → 2hno 3 (aq) when the clouds. 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3. Nitrogen Oxide React With Water.
From www.tessshebaylo.com
Ammonia Gas And Water Equation Tessshebaylo Nitrogen Oxide React With Water 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. Nitrogen dioxide concentration in unpolluted air is around 10 parts per. Nitrogen Oxide React With Water.
From www.teachoo.com
[MCQ] Which of the statements is not correct? All metal oxides react Nitrogen Oxide React With Water Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble in water (therefore, nitric acid exists as an. 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Alkali. Nitrogen Oxide React With Water.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Nitrogen Oxide React With Water Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble in water (therefore, nitric acid exists as an. Nitrogen dioxide concentration in unpolluted air is around 10 parts. Nitrogen Oxide React With Water.
From www.numerade.com
SOLVEDNitrogen oxide (NO) has been found to be a key component in many Nitrogen Oxide React With Water Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid in acid rain. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble in water (therefore, nitric acid exists as an. 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3. Nitrogen Oxide React With Water.
From www.tessshebaylo.com
Write The Chemical Equation Of Mixing Ammonia Nh3 With Water Tessshebaylo Nitrogen Oxide React With Water No 2 (aq) + h 2 o (l) + 1½o 2 (g) → 2hno 3 (aq) when the clouds. Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid in acid rain. Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion. The nitrogen(iv) oxide (no 2) dissolves. Nitrogen Oxide React With Water.
From www.numerade.com
SOLVED Nitrogen dioxide (NO2) gas and liquid water (H2O) react to form Nitrogen Oxide React With Water Oxides of group 1 elements also react with water to create basic solutions. 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. No 2 (aq) + h 2 o (l) + 1½o 2 (g) → 2hno 3 (aq) when the clouds. Alkali metals react with oxygen to. Nitrogen Oxide React With Water.
From www.vecteezy.com
Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide Nitrogen Oxide React With Water Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. Oxides of group 1 elements also react with water to create basic solutions. Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid. Nitrogen Oxide React With Water.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas Nitric Oxide Gas Reacts With Hydrogen Gas Nitrogen Oxide React With Water 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. Oxides of group 1 elements also react with water to create basic solutions. So 3 reacts with water as follows: Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion. Nitrogen dioxide in the air also. Nitrogen Oxide React With Water.
From www.chemistrystudent.com
Organic Quick Notes (revision) for A2Level ChemistryStudent Nitrogen Oxide React With Water Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Oxides of group 1 elements also react with water to create basic solutions. So 3 reacts with water as. Nitrogen Oxide React With Water.
From www.alamy.com
Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide Nitrogen Oxide React With Water The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Oxides of group 1 elements also react with water to create basic solutions. Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Nitrogen dioxide in the air also reacts. Nitrogen Oxide React With Water.
From www.numerade.com
SOLVEDNitrogen oxide (NO) has been found to be a key component in many Nitrogen Oxide React With Water The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Alkali metals react with oxygen to form monoxides, peroxides, or superoxides. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble in water (therefore, nitric acid exists as an. No 2 (aq) + h 2 o (l) + 1½o 2 (g). Nitrogen Oxide React With Water.
From www.chegg.com
Solved Nitrogen monoxide and oxygen react to form nitrogen Nitrogen Oxide React With Water Oxides of group 1 elements also react with water to create basic solutions. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: So 3 reacts with water as follows: The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: 2no 2 (aq) + h 2 o(l) + ½o 2 (g) →. Nitrogen Oxide React With Water.
From a-z-animals.com
Discover the Molar Mass of Nitrogen (N) + How It Compares to Other Nitrogen Oxide React With Water Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble in water (therefore, nitric acid exists as an. So 3 reacts with water as follows: Alkali metals react with oxygen to form monoxides, peroxides, or superoxides. Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and. Nitrogen Oxide React With Water.
From www.numerade.com
SOLVED Nitrous acid (HNO2) disproportionates in acidic solutionto Nitrogen Oxide React With Water Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid in acid rain. So 3 reacts with water as follows: The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: No 2 (aq) + h 2 o (l) + 1½o 2 (g) → 2hno 3 (aq). Nitrogen Oxide React With Water.
From astonishingceiyrs.blogspot.com
Sodium Oxide Reacts With Water astonishingceiyrs Nitrogen Oxide React With Water Oxides of group 1 elements also react with water to create basic solutions. So 3 reacts with water as follows: Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as. Nitrogen Oxide React With Water.
From chemistryguru.com.sg
Reaction of Period 3 Oxides with Water, Acids and Bases Nitrogen Oxide React With Water Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion. Oxides of group 1 elements also react with water to create basic solutions. Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. The nitrogen(iv) oxide (no 2) dissolves and reacts. Nitrogen Oxide React With Water.
From www.doubtnut.com
Nitric oxide reacts with hydrogen to give nitrogen and water 2NO +2H2 Nitrogen Oxide React With Water The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of. Nitrogen Oxide React With Water.
From www.numerade.com
SOLVEDproducts are formed, one of which is Benzene, CoH6, reacts with Nitrogen Oxide React With Water Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Oxides of group 1 elements also react with water to create basic solutions. So 3 reacts with water as. Nitrogen Oxide React With Water.
From askfilo.com
Metals react with water and produce a metal oxide and hydrogen gas. Metal.. Nitrogen Oxide React With Water The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble in water (therefore, nitric acid exists as an. 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. So. Nitrogen Oxide React With Water.
From www.numerade.com
SOLVED Nitrogen dioxide gas can react with water to form nitric acid Nitrogen Oxide React With Water Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion. Alkali metals react with oxygen to form monoxides, peroxides, or superoxides. Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. So 3 reacts with water as follows: No 2 (aq). Nitrogen Oxide React With Water.
From kunduz.com
[ANSWERED] 1 Nitrogen dioxide and water react to produce nitric acid Nitrogen Oxide React With Water Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble in water (therefore, nitric acid exists as an. So 3 reacts with water as follows: Oxides of group 1 elements also react with water to create basic solutions. Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion. No 2 (aq) + h. Nitrogen Oxide React With Water.
From www.numerade.com
SOLVEDNitrogen dioxide and water react to produce nitric acid, HNO3 Nitrogen Oxide React With Water So 3 reacts with water as follows: 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. No 2 (aq) + h 2 o (l) + 1½o 2 (g) → 2hno 3 (aq) when the clouds. Nitrogen dioxide in the air also reacts with water vapor to form. Nitrogen Oxide React With Water.
From www.hanlin.com
CIE A Level Chemistry复习笔记2.1.3 Period 3 Oxides翰林国际教育 Nitrogen Oxide React With Water Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid in acid rain. Although nitric oxide is thermodynamically unstable, it is kinetically stable as. Nitrogen Oxide React With Water.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas And Oxygen Gas Reaction Nitrogen Oxide React With Water No 2 (aq) + h 2 o (l) + 1½o 2 (g) → 2hno 3 (aq) when the clouds. Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid in acid rain. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: 2no 2 (aq) +. Nitrogen Oxide React With Water.
From www.numerade.com
SOLVED Nitrogen dioxide and water react to produce nitric acid, HNO3 Nitrogen Oxide React With Water 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid in acid rain. The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Alkali. Nitrogen Oxide React With Water.
From www.youtube.com
Chemical Reaction of Water with Nitrous Oxide, Chemistry Lecture Nitrogen Oxide React With Water The nitrogen(iv) oxide (no 2) dissolves and reacts in water with oxygen as follows: Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. So 3 reacts with water as follows: No 2 (aq) + h 2 o (l) + 1½o 2 (g). Nitrogen Oxide React With Water.
From cason-blogbates.blogspot.com
Nitrogen Dioxide Reacts With Water to Form Nitric Acid Nitrogen Oxide React With Water Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. Gaseous nitrogen dioxide reacts with liquid water and produce nitric acid which is highly soluble in water (therefore, nitric acid exists as an. Nitrogen dioxide concentration in unpolluted air is around 10 parts. Nitrogen Oxide React With Water.
From www.chegg.com
Solved 6. When two volumes of hydrogen gas react with one Nitrogen Oxide React With Water Oxides of group 1 elements also react with water to create basic solutions. So 3 reacts with water as follows: Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion. Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid in acid rain. No 2 (aq) + h. Nitrogen Oxide React With Water.
From www.numerade.com
SOLVEDNitrogen dioxide and water react to produce nitric acid, HNO3 Nitrogen Oxide React With Water Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. 2no 2 (aq) + h 2 o(l) + ½o 2 (g) → 2hno 3 (aq) when the clouds rise, the temperature decreases,. Oxides of group 1 elements also react with water to create. Nitrogen Oxide React With Water.
From www.numerade.com
SOLVEDAmmonia reacts with oxygen to yield nitrogen and water. 4 NH3(g Nitrogen Oxide React With Water Although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and so it is considered stable and won't normally. So 3 reacts with water as follows: Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion. Alkali metals react with oxygen to form monoxides, peroxides, or superoxides. No 2 (aq). Nitrogen Oxide React With Water.