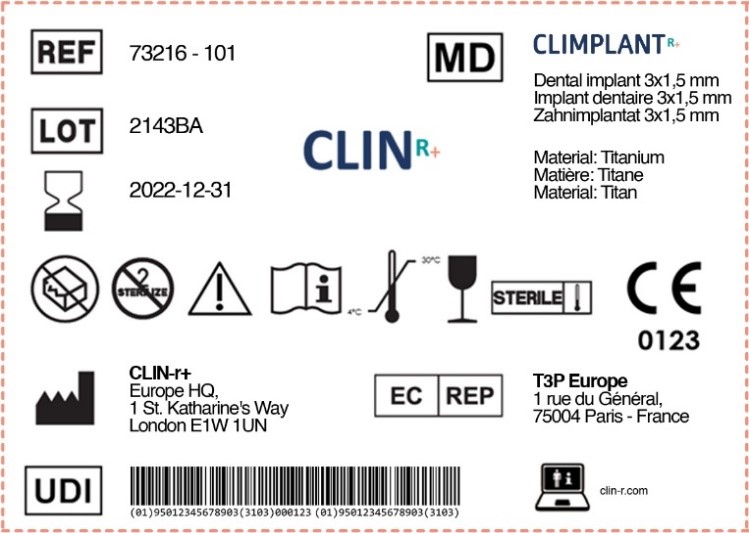
Fda Guidance On Medical Device Patient Labeling Warnings And Precautions . The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. It is intended to help assure that. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. As explained by the fda, they should include the following. Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling.
from www.vrogue.co
This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. It is intended to help assure that. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. As explained by the fda, they should include the following.
Fda Medical Device Label Symbols vrogue.co
Fda Guidance On Medical Device Patient Labeling Warnings And Precautions The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. As explained by the fda, they should include the following. It is intended to help assure that. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,.
From docslib.org
Guidance on Medical Device Patient Labeling; Final Guidance for Industry and FDA Reviewers DocsLib Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. As explained by the fda, they should include. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From slideplayer.com
PFO FDA Considerations for Labeling and Future Trials ppt download Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. It is intended to help assure that. Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. This. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.linkedin.com
GUIDELINES FOR EFFECTIVE AND COMPLIANT MEDICAL DEVICE LABELING ENSURING PATIENT SAFETY AND Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. As explained by the fda, they should include the following. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. The. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Fda Guidance On Medical Device Patient Labeling Warnings And Precautions This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. As explained by the fda, they should include the following. This guidance is intended to assist applicants and reviewers in drafting the. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Troubleshooting and Additional Information Fda Guidance On Medical Device Patient Labeling Warnings And Precautions The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. The fda's guidance on medical device labeling suggests several. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From mavink.com
Medical Device Labeling Symbols Fda Guidance On Medical Device Patient Labeling Warnings And Precautions The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. As explained by the fda, they should include the following. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Warnings and Precautions RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions It is intended to help assure that. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. As explained by the fda, they should include the following. The fda's guidance on medical device labeling suggests several strategies. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on Patient Labeling Appearance of Text and Graphics RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. Consistent with suggestions in the fda's guidance document. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Fda Guidance On Medical Device Patient Labeling Warnings And Precautions As explained by the fda, they should include the following. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. This guidance is intended to assist applicants and reviewers in drafting the warnings and. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Troubleshooting and Additional Information Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. It is intended to help assure that. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. This guidance. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Readability RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. It is intended to help assure that. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. Medical device patient labeling is any information associated with a device targeted to the patient or lay. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.ericshaver.com
Design Best Practices for Medical Device Labeling Warnings Eric Shaver Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. It is intended to help assure that. The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. This. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Warnings and Precautions RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. It is intended to help assure that. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. Consistent with. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From emmainternational.com
Understanding FDA Guidance Documents Fda Guidance On Medical Device Patient Labeling Warnings And Precautions The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. As explained by the fda, they should include the following. Consistent with suggestions in the fda's guidance document on medical device patient labeling,. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Pretesting RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions As explained by the fda, they should include the following. The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. It is intended to help assure that. Medical device patient labeling is any information associated with. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. It is intended to help assure that. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. The fda's guidance on. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Fda Guidance On Medical Device Patient Labeling Warnings And Precautions This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. As explained by the fda, they should include the following. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Overview RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. It is intended to help assure that. The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From pdfslide.net
(PDF) Guidance on Medical Device Patient Labeling; FInal … on Medical Device Patient Labeling Fda Guidance On Medical Device Patient Labeling Warnings And Precautions This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. The fda's guidance on medical device labeling suggests several. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. It is intended to help assure that. Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. This. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Operational Information RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. As explained by the fda, they should include the following. It is intended to help assure that. The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. The guidance also describes the content of warnings and. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Specific Aspects RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. As explained by the fda, they should include the following. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. The guidance also describes the content of warnings and precautions to be included in. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. The fda's guidance on. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From medicalxpress.com
FDA drafts guidance on patient labeling information for LASIK devices Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. It is intended to help. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. It is intended to help assure that. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.uslegalforms.com
FDA Patient Labeling 101 Fill and Sign Printable Template Online US Legal Forms Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. As explained by the fda, they should include the. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions As explained by the fda, they should include the following. It is intended to help assure that. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. Consistent with suggestions in the. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.youtube.com
FDA Requirements for Device Labeling YouTube Fda Guidance On Medical Device Patient Labeling Warnings And Precautions As explained by the fda, they should include the following. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. This guidance assists manufacturers in their development, and assist center reviewers in their review and. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. The fda's guidance on medical device. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Guidance On Medical Device Patient Labeling Warnings And Precautions This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient. It is intended to help assure that. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. Medical device patient labeling is any information associated with a device targeted to the patient or lay. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Symbol Use Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. The fda's guidance on medical device labeling suggests several strategies for effective labeling, including integrating warnings and precautions into. As explained by the fda, they should include the following. Consistent with suggestions in the fda's guidance document on medical device patient labeling,. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Registration & Labeling Fda Guidance On Medical Device Patient Labeling Warnings And Precautions The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. It is intended to help assure that. This guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical device patient.. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From mungfali.com
FDA Medical Device Label Symbols Fda Guidance On Medical Device Patient Labeling Warnings And Precautions The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. It is intended to help assure that. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. The fda's. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Overview RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. This guidance is intended to assist applicants and reviewers in drafting the warnings and precautions,. Consistent with suggestions in the fda's guidance document on. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Fda Guidance On Medical Device Patient Labeling Warnings And Precautions The guidance also describes the content of warnings and precautions to be included in medical device patient labeling. Medical device patient labeling is any information associated with a device targeted to the patient or lay caregiver. It is intended to help assure that. Consistent with suggestions in the fda's guidance document on medical device patient labeling, include critical information. As. Fda Guidance On Medical Device Patient Labeling Warnings And Precautions.