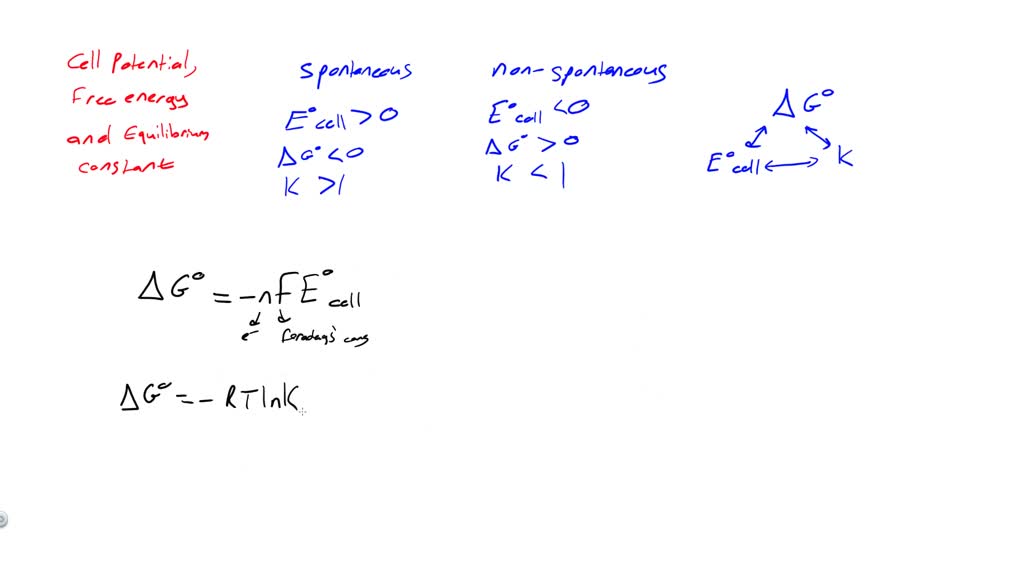
Equilibrium Constant In Electrochemistry . The standard state potential for a cell. If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's constant, and the reaction quotient. Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. At equilibrium, the reaction quotient is the equilibrium constant. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. The nernst equation is used in. To calculate the equilibrium constant for an electrochemical cell we need to know:
from www.numerade.com
In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. At equilibrium, the reaction quotient is the equilibrium constant. To calculate the equilibrium constant for an electrochemical cell we need to know: The standard state potential for a cell. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. The nernst equation is used in. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's constant, and the reaction quotient.
Electrochemistry intro Numerade
Equilibrium Constant In Electrochemistry To calculate the equilibrium constant for an electrochemical cell we need to know: To calculate the equilibrium constant for an electrochemical cell we need to know: If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The nernst equation is used in. The standard state potential for a cell. At equilibrium, the reaction quotient is the equilibrium constant. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's constant, and the reaction quotient.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Equilibrium Constant In Electrochemistry At equilibrium, the reaction quotient is the equilibrium constant. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's constant, and the reaction quotient. To calculate the equilibrium constant for. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Electrochemistry 10 Relationship between Nernst Equation and Equilibrium Constant In Electrochemistry The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. To calculate the equilibrium constant for an electrochemical cell we need to know: In this section we will introduce the electrochemical potential into the gibbs. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Relation Between EMF and Equilibrium Constant Class 12 Chemistry Equilibrium Constant In Electrochemistry If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's constant, and the reaction quotient. At equilibrium, the reaction quotient is the equilibrium constant. The standard state potential for a cell.. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Relationship between Gibbs free energy, EMF of cell and Equilibrium Equilibrium Constant In Electrochemistry Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. The standard state potential for a cell. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. At equilibrium, the reaction quotient is the equilibrium constant. To calculate the equilibrium constant for. Equilibrium Constant In Electrochemistry.
From slideplayer.com
Electrochemistry. ppt download Equilibrium Constant In Electrochemistry The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's constant, and the reaction quotient. In this section we will introduce the electrochemical potential into the gibbs potential and discuss. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Class 12 Electrochemistry ( How to calculate equilibrium constant by Equilibrium Constant In Electrochemistry If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of. Equilibrium Constant In Electrochemistry.
From www.youtube.com
3.6Equilibrium constant from Nernst equation, class 12th Equilibrium Constant In Electrochemistry If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. At equilibrium, the reaction quotient is the equilibrium constant. The nernst equation is used in. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The standard state potential. Equilibrium Constant In Electrochemistry.
From www.youtube.com
3.8Relationship between Gibbs free energy and equilibrium constant Equilibrium Constant In Electrochemistry To calculate the equilibrium constant for an electrochemical cell we need to know: The standard state potential for a cell. The nernst equation is used in. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The nernst equation calculates electrochemical cell potential from standard cell. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Electrochemistry Part6 Equilibrium Constant for Redox Equations YouTube Equilibrium Constant In Electrochemistry At equilibrium, the reaction quotient is the equilibrium constant. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. To calculate the equilibrium constant for an electrochemical cell we need to know: If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. The standard state. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Equilibrium constant from daniel cell (Electrochemistry part 31 for Equilibrium Constant In Electrochemistry The standard state potential for a cell. At equilibrium, the reaction quotient is the equilibrium constant. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The nernst. Equilibrium Constant In Electrochemistry.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2851831 Equilibrium Constant In Electrochemistry The nernst equation is used in. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. At equilibrium, the reaction quotient is the equilibrium constant. To calculate the. Equilibrium Constant In Electrochemistry.
From itsreleased.com
Nernst Equation Understanding Electrochemical Equilibrium Equilibrium Constant In Electrochemistry The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's constant, and the reaction quotient. At equilibrium, the reaction quotient is the equilibrium constant. To calculate the equilibrium constant for an electrochemical cell we need to know: The correlation between cell potential (e), free energy (δg), and equilibrium. Equilibrium Constant In Electrochemistry.
From www.youtube.com
18.8 Equilibrium Constants for Galvanic Cells YouTube Equilibrium Constant In Electrochemistry The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. The standard state potential for a cell. If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze. Equilibrium Constant In Electrochemistry.
From www.slideserve.com
PPT Electrochemistry MAE 212 PowerPoint Presentation, free download Equilibrium Constant In Electrochemistry The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. To calculate the equilibrium constant for an electrochemical cell we need to know: The standard state potential for a cell. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. Equilibrium Constant In Electrochemistry.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Equilibrium Constant In Electrochemistry The standard state potential for a cell. To calculate the equilibrium constant for an electrochemical cell we need to know: Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of. Equilibrium Constant In Electrochemistry.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free Equilibrium Constant In Electrochemistry If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. At equilibrium, the reaction quotient is the equilibrium constant. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The nernst equation calculates electrochemical cell potential from standard cell. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Electrochemistry Equilibrium constant class 12 YouTube Equilibrium Constant In Electrochemistry If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. To calculate the equilibrium constant for an electrochemical cell we need to know: At equilibrium, the reaction quotient is the equilibrium constant. The. Equilibrium Constant In Electrochemistry.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Equilibrium Constant In Electrochemistry At equilibrium, the reaction quotient is the equilibrium constant. To calculate the equilibrium constant for an electrochemical cell we need to know: The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. The standard state potential for a cell. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Electrochemistry and Free Energy, Nernst Equation, Equilibrium Constant Equilibrium Constant In Electrochemistry If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. To calculate the equilibrium constant for an electrochemical cell we need to know: At equilibrium, the reaction quotient is the equilibrium constant. Use the data. Equilibrium Constant In Electrochemistry.
From www.toppr.com
Given the equilibrium constant, Kc of the reaction Cu(s) + 2Ag^ + (aq Equilibrium Constant In Electrochemistry The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. The nernst equation is used in. At equilibrium, the reaction quotient is the equilibrium constant. The standard state potential for a cell. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of. Equilibrium Constant In Electrochemistry.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent Equilibrium Constant In Electrochemistry If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. At equilibrium, the reaction quotient is the equilibrium constant. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's constant, and the reaction quotient. To calculate the equilibrium constant for an. Equilibrium Constant In Electrochemistry.
From slideplayer.com
Chapter 17 Electrochemistry ppt download Equilibrium Constant In Electrochemistry The standard state potential for a cell. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. At equilibrium, the reaction quotient is the equilibrium constant.. Equilibrium Constant In Electrochemistry.
From www.youtube.com
ELECTROCHEMISTRY L5 EQUILIBRIUM CONSTANT FROM NERNST EQUATION AND Equilibrium Constant In Electrochemistry The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. The nernst equation is used in. To calculate the equilibrium constant for an electrochemical cell we need to know: The standard state potential for a cell. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches. Equilibrium Constant In Electrochemistry.
From www.youtube.com
LECTURE 8 ELECTROCHEMISTRY NERST EQUATION and EQUILIBRIUM CONSTANT Equilibrium Constant In Electrochemistry At equilibrium, the reaction quotient is the equilibrium constant. If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are. Equilibrium Constant In Electrochemistry.
From in.pinterest.com
Nernst equation. ️Relationship between EMF And Equilibrium constant. 🤟 Equilibrium Constant In Electrochemistry To calculate the equilibrium constant for an electrochemical cell we need to know: The standard state potential for a cell. At equilibrium, the reaction quotient is the equilibrium constant. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to analyze electrochemical reactions by measurement of electrical. The nernst equation is used in. The. Equilibrium Constant In Electrochemistry.
From app.jove.com
Gibbs Free Energy, Cell Potential and Equilibrium Constant Chemistry Equilibrium Constant In Electrochemistry The standard state potential for a cell. Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. To calculate the equilibrium constant for an electrochemical cell we need to know: If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. In this section. Equilibrium Constant In Electrochemistry.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Equilibrium Constant In Electrochemistry Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. The standard state potential for. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Problem 3 on Gibbs Free Energy &equilibrium constant(Electrochemistry Equilibrium Constant In Electrochemistry The standard state potential for a cell. At equilibrium, the reaction quotient is the equilibrium constant. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. If thermodynamic variables are temperature and. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Electrochemistry 5 Nernst Equation Equilibrium Constant Gibb’s Equilibrium Constant In Electrochemistry The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. To calculate the equilibrium constant for an electrochemical cell we need to know: Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. The standard state potential for a cell. In this. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Electrochemistry chemistry numerical 13 Calculate std. Gibbs energy Equilibrium Constant In Electrochemistry If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. To calculate the equilibrium constant for an electrochemical cell we need to know: The standard state potential for a cell. The nernst equation is used in. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number. Equilibrium Constant In Electrochemistry.
From www.numerade.com
Electrochemistry intro Numerade Equilibrium Constant In Electrochemistry The standard state potential for a cell. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's constant, and the reaction quotient. At equilibrium, the reaction quotient is the equilibrium constant. The nernst equation is used in. To calculate the equilibrium constant for an electrochemical cell we need. Equilibrium Constant In Electrochemistry.
From mrschimomot.blogspot.com
Gibbs Free Energy Formula In Electrochemistry Mrschimomot Equilibrium Constant In Electrochemistry To calculate the equilibrium constant for an electrochemical cell we need to know: At equilibrium, the reaction quotient is the equilibrium constant. If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. The nernst equation. Equilibrium Constant In Electrochemistry.
From www.studocu.com
Electrochemistry electrochem Example Calculate the equilibrium Equilibrium Constant In Electrochemistry To calculate the equilibrium constant for an electrochemical cell we need to know: The nernst equation is used in. The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's constant,. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Electrochemistry Nernst Equation & Equilibrium Constant YouTube Equilibrium Constant In Electrochemistry At equilibrium, the reaction quotient is the equilibrium constant. The nernst equation is used in. If thermodynamic variables are temperature and pressure, the main chemical parameter for determining whether a system or. Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. The nernst equation calculates electrochemical cell potential from. Equilibrium Constant In Electrochemistry.
From www.youtube.com
Electrochemistry Part5 Class +2 Unit3 Nernst Equation Equilibrium Constant In Electrochemistry To calculate the equilibrium constant for an electrochemical cell we need to know: The correlation between cell potential (e), free energy (δg), and equilibrium constant (k) are given by important equations. Use the data in table p2 to calculate the equilibrium constant for the reaction of \(\ce{sn^{2+}(aq)}\) with oxygen to produce. The nernst equation is used in. The nernst equation. Equilibrium Constant In Electrochemistry.