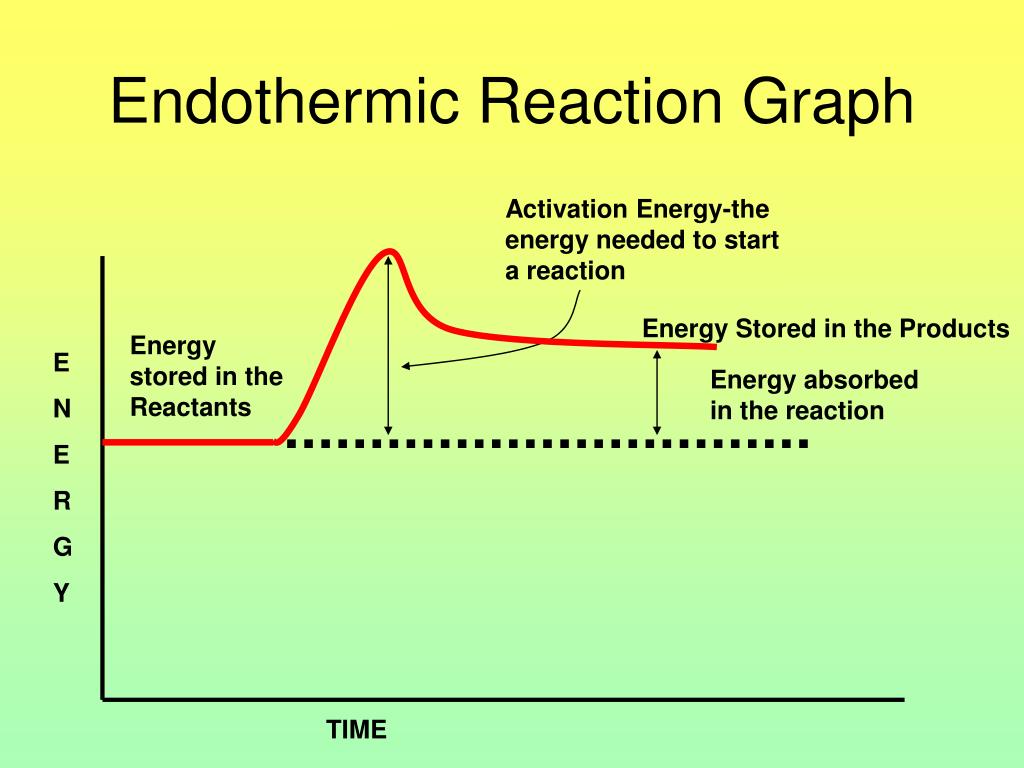
Endothermic Reaction Exothermic Reaction . Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. As such there is a release of energy as we move from reactants to. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The energy level decreases in an exothermic reaction. This is because energy is given out to the surroundings. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. Because heat is absorbed, endothermic reactions feel cold. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their.
from www.slideserve.com
As such there is a release of energy as we move from reactants to. This is because energy is given out to the surroundings. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. Some examples of endothermic reactions are: The energy level decreases in an exothermic reaction. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. The reaction between ethanoic acid and sodium carbonate. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their.
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint
Endothermic Reaction Exothermic Reaction The energy level decreases in an exothermic reaction. The reaction between ethanoic acid and sodium carbonate. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The energy level decreases in an exothermic reaction. This is because energy is given out to the surroundings. Because heat is absorbed, endothermic reactions feel cold. As such there is a release of energy as we move from reactants to. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. Some examples of endothermic reactions are:
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Reaction Exothermic Reaction Because heat is absorbed, endothermic reactions feel cold. This is because energy is given out to the surroundings. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Revise and understand what endothermic and exothermic reactions are. Endothermic Reaction Exothermic Reaction.
From www.youtube.com
Endothermic and Exothermic Reactions With Potential Energy Diagrams Endothermic Reaction Exothermic Reaction Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. This is because energy is given out to the surroundings. The energy level decreases in an exothermic reaction. As such there is a release of. Endothermic Reaction Exothermic Reaction.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Endothermic Reaction Exothermic Reaction The energy level decreases in an exothermic reaction. As such there is a release of energy as we move from reactants to. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. Because heat is absorbed, endothermic reactions feel cold. Some examples of endothermic reactions are: This is because energy is. Endothermic Reaction Exothermic Reaction.
From www.youtube.com
Exothermic vs Endothermic Chemical Reactions YouTube Endothermic Reaction Exothermic Reaction An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. This is because energy is given out to the surroundings. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. As such there is a release of. Endothermic Reaction Exothermic Reaction.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Exothermic Reaction Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. The energy level decreases in an exothermic reaction. Because heat is absorbed, endothermic reactions feel cold. This is because energy is given out to the surroundings. The reaction between ethanoic acid and sodium carbonate. As such there is a. Endothermic Reaction Exothermic Reaction.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Exothermic Reaction The reaction between ethanoic acid and sodium carbonate. This is because energy is given out to the surroundings. As such there is a release of energy as we move from reactants to. The energy level decreases in an exothermic reaction. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions. Endothermic Reaction Exothermic Reaction.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Exothermic Reaction An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. As such there is a release of energy as we move from reactants to. This is because energy is given out to the surroundings. Because heat is absorbed, endothermic reactions feel cold. The energy level decreases in an exothermic reaction. Revise and understand what endothermic and. Endothermic Reaction Exothermic Reaction.
From www.youtube.com
Chemical Reaction and equation (Exothermic and Endothermic Reaction Endothermic Reaction Exothermic Reaction Because heat is absorbed, endothermic reactions feel cold. As such there is a release of energy as we move from reactants to. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. This is because energy is given out to the surroundings. Reactions are exothermic when the energy stored. Endothermic Reaction Exothermic Reaction.
From www.youtube.com
Endothermic & Exothermic reaction class 10 chemical reaction and Endothermic Reaction Exothermic Reaction As such there is a release of energy as we move from reactants to. This is because energy is given out to the surroundings. Because heat is absorbed, endothermic reactions feel cold. Some examples of endothermic reactions are: The energy level decreases in an exothermic reaction. Reactions are exothermic when the energy stored by the reactants is more than the. Endothermic Reaction Exothermic Reaction.
From byjus.com
24. What is the Minimum activation energy required for endothermic and Endothermic Reaction Exothermic Reaction As such there is a release of energy as we move from reactants to. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the. Endothermic Reaction Exothermic Reaction.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Exothermic Reaction Because heat is absorbed, endothermic reactions feel cold. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. The energy level decreases in an exothermic reaction. Reactions are exothermic when the energy stored by the reactants is more than the energy stored. Endothermic Reaction Exothermic Reaction.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Exothermic Reaction An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. The reaction between ethanoic acid and sodium carbonate. Because heat is absorbed, endothermic reactions feel cold. Reactions are exothermic when the energy stored by the. Endothermic Reaction Exothermic Reaction.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation Endothermic Reaction Exothermic Reaction The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. This is because energy is given out to the surroundings. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Some examples of endothermic reactions are: Because. Endothermic Reaction Exothermic Reaction.
From gy.kimiq.com
In An Exothermic Reaction Energy Is Transferred From Energy Etfs Endothermic Reaction Exothermic Reaction Some examples of endothermic reactions are: This is because energy is given out to the surroundings. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. The reaction between ethanoic acid and sodium carbonate. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer. Endothermic Reaction Exothermic Reaction.
From www.pinterest.co.uk
Endothermic and Exothermic Reactions Lab ⋆ Exothermic Endothermic Reaction Exothermic Reaction This is because energy is given out to the surroundings. As such there is a release of energy as we move from reactants to. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical reaction that absorbs. Endothermic Reaction Exothermic Reaction.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction Exothermic Reaction Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. This is because energy is given out to the surroundings. Reactions are exothermic when the energy stored by the reactants is more than the energy. Endothermic Reaction Exothermic Reaction.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction Exothermic Reaction Some examples of endothermic reactions are: An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. The energy level decreases in an exothermic reaction. As such there is a release of energy as we move from reactants to. Revise and understand what endothermic and exothermic reactions are and. Endothermic Reaction Exothermic Reaction.
From question.pandai.org
Endothermic and exothermic reactions Endothermic Reaction Exothermic Reaction An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The reaction between ethanoic acid and sodium carbonate. The energy level decreases in an exothermic reaction. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Some examples of endothermic reactions are: This is because. Endothermic Reaction Exothermic Reaction.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Exothermic Reaction This is because energy is given out to the surroundings. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The energy level decreases in an exothermic reaction. Some examples of endothermic reactions are: Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. As such there. Endothermic Reaction Exothermic Reaction.
From www.yaclass.in
Exothermic and Endothermic chemical changes — lesson. Science State Endothermic Reaction Exothermic Reaction This is because energy is given out to the surroundings. The reaction between ethanoic acid and sodium carbonate. Some examples of endothermic reactions are: As such there is a release of energy as we move from reactants to. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold.. Endothermic Reaction Exothermic Reaction.
From www.youtube.com
Endothermic Vs. Exothermic Reaction Graphs YouTube Endothermic Reaction Exothermic Reaction This is because energy is given out to the surroundings. The reaction between ethanoic acid and sodium carbonate. Some examples of endothermic reactions are: An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. As such there is a release of energy as we move from reactants to. The energy level decreases in an exothermic reaction.. Endothermic Reaction Exothermic Reaction.
From pixels.com
Exothermic And Endothermic Chemical Reactions Photograph by Inna Bigun Endothermic Reaction Exothermic Reaction The reaction between ethanoic acid and sodium carbonate. As such there is a release of energy as we move from reactants to. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. This is because energy is given out to the surroundings. Revise and understand what endothermic and exothermic reactions are and how the two reactions. Endothermic Reaction Exothermic Reaction.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Exothermic Reaction Because heat is absorbed, endothermic reactions feel cold. The energy level decreases in an exothermic reaction. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. This is because energy is given out to the surroundings. Some examples of endothermic reactions are: An endothermic reaction is a chemical reaction. Endothermic Reaction Exothermic Reaction.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Exothermic Reaction An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The energy level decreases in an exothermic reaction. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. The reaction between ethanoic acid and sodium carbonate. Because heat is absorbed, endothermic reactions feel cold. Some. Endothermic Reaction Exothermic Reaction.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Exothermic Reaction The reaction between ethanoic acid and sodium carbonate. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Because heat is absorbed, endothermic reactions feel cold. Some examples of endothermic reactions are: The energy level decreases in an exothermic reaction. This is because energy is given out to the. Endothermic Reaction Exothermic Reaction.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Endothermic Reaction Exothermic Reaction An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. Some examples of endothermic reactions are: This is because energy is given out to the surroundings. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. As. Endothermic Reaction Exothermic Reaction.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Endothermic Reaction Exothermic Reaction An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. The reaction between ethanoic acid and sodium carbonate. As such there is a release of energy as we move from reactants to. This is because energy is. Endothermic Reaction Exothermic Reaction.
From 5differencebetween.com
5 Difference Between Endothermic and Exothermic Reaction Endothermic Endothermic Reaction Exothermic Reaction An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. This is because energy is given out to the surroundings. Some examples of endothermic reactions are: Because heat is absorbed, endothermic reactions feel cold. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. As such there. Endothermic Reaction Exothermic Reaction.
From www.scribd.com
Endothermic Reaction Exothermic Reaction Delta H Delta H PDF Endothermic Reaction Exothermic Reaction Because heat is absorbed, endothermic reactions feel cold. This is because energy is given out to the surroundings. The reaction between ethanoic acid and sodium carbonate. The energy level decreases in an exothermic reaction. Some examples of endothermic reactions are: An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Reactions are exothermic when the energy. Endothermic Reaction Exothermic Reaction.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Reaction Exothermic Reaction This is because energy is given out to the surroundings. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. As such there is a release of energy as we move from reactants to. An. Endothermic Reaction Exothermic Reaction.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube Endothermic Reaction Exothermic Reaction This is because energy is given out to the surroundings. The reaction between ethanoic acid and sodium carbonate. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Some examples. Endothermic Reaction Exothermic Reaction.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction Exothermic Reaction The energy level decreases in an exothermic reaction. Because heat is absorbed, endothermic reactions feel cold. This is because energy is given out to the surroundings. The reaction between ethanoic acid and sodium carbonate. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. As such there is a. Endothermic Reaction Exothermic Reaction.
From kunduz.com
Exothermic Reaction Definition, Properties, Examples, Exothermic and Endothermic Reaction Exothermic Reaction The reaction between ethanoic acid and sodium carbonate. The energy level decreases in an exothermic reaction. As such there is a release of energy as we move from reactants to. This is because energy is given out to the surroundings. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Revise and understand what endothermic and. Endothermic Reaction Exothermic Reaction.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Endothermic Reaction Exothermic Reaction As such there is a release of energy as we move from reactants to. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Some examples of endothermic reactions are: Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. The energy level decreases in an exothermic. Endothermic Reaction Exothermic Reaction.
From askanydifference.com
Exothermic vs Endothermic Reaction Difference and Comparison Endothermic Reaction Exothermic Reaction Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. The energy level decreases in an exothermic reaction. As such there is a release of energy as we move from. Endothermic Reaction Exothermic Reaction.