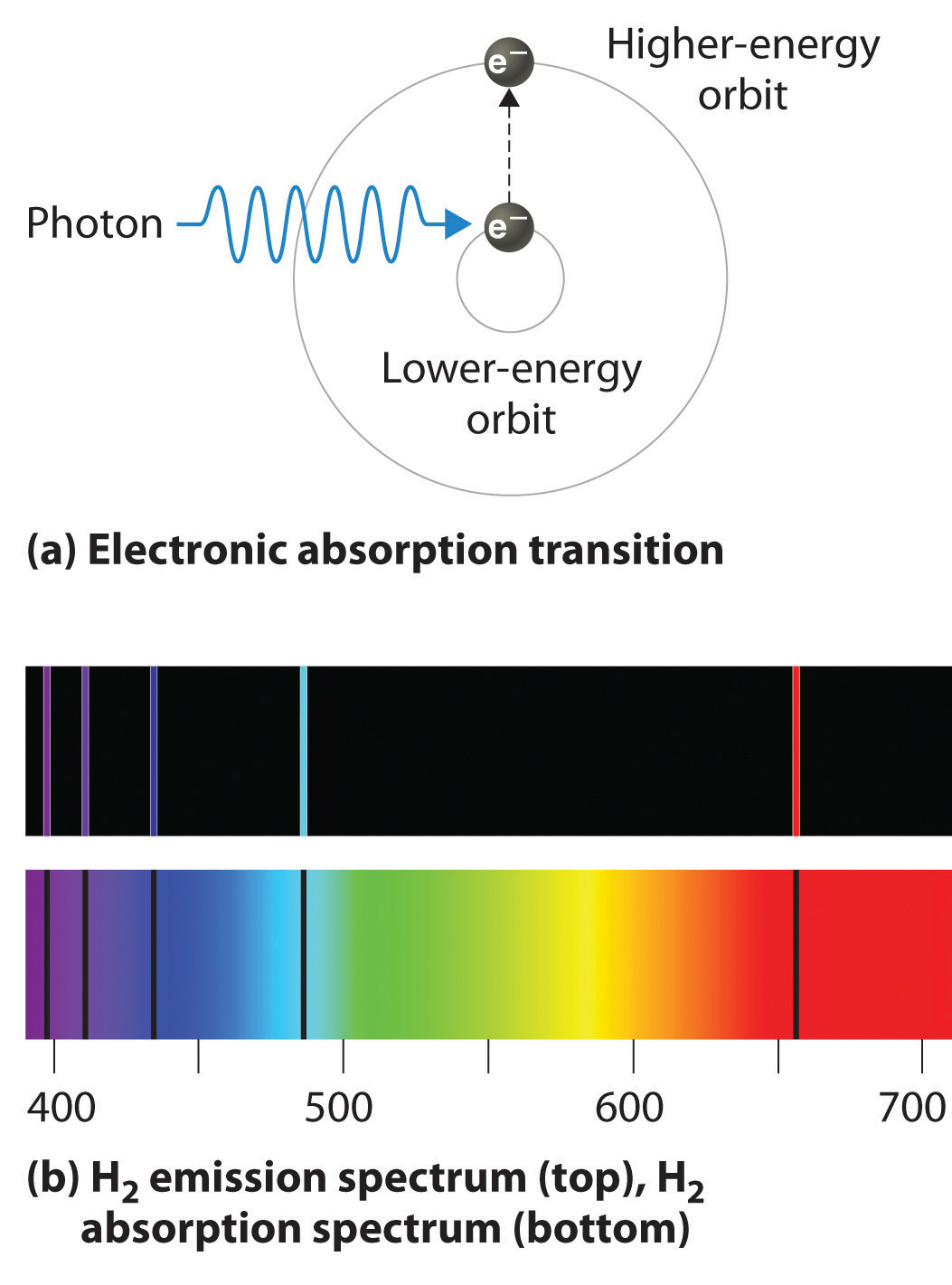
Electromagnetic Spectrum Vs Atomic Emission Spectrum . this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. emission spectrum (or atomic spectrum): the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the longest wavelength/lowest. let us understand the phenomenon of dispersion of white light through a prism. To be in the lowest energy. Also, learn about the e mission spectrum and absorption spectrum. using a bohr model and the transition from n = 2 to n = 3 in an atom with a single electron, describe the mathematical relationship. The unique pattern of light given off by an element when it is given energy;
from chem.libretexts.org
using a bohr model and the transition from n = 2 to n = 3 in an atom with a single electron, describe the mathematical relationship. the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the longest wavelength/lowest. To be in the lowest energy. emission spectrum (or atomic spectrum): an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. Also, learn about the e mission spectrum and absorption spectrum. this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The unique pattern of light given off by an element when it is given energy; let us understand the phenomenon of dispersion of white light through a prism.
Chapter 2.3 Atomic Spectra and Models of the Atom Chemistry LibreTexts
Electromagnetic Spectrum Vs Atomic Emission Spectrum emission spectrum (or atomic spectrum): this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. To be in the lowest energy. Also, learn about the e mission spectrum and absorption spectrum. the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the longest wavelength/lowest. The unique pattern of light given off by an element when it is given energy; let us understand the phenomenon of dispersion of white light through a prism. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. using a bohr model and the transition from n = 2 to n = 3 in an atom with a single electron, describe the mathematical relationship. emission spectrum (or atomic spectrum): atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample.
From www.differencebetween.com
Difference Between Atomic Absorption and Atomic Emission Compare the Electromagnetic Spectrum Vs Atomic Emission Spectrum an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. let us understand the phenomenon of dispersion of white light through a prism. the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Electromagnetic Spectrum Vs Atomic Emission Spectrum Also, learn about the e mission spectrum and absorption spectrum. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the longest wavelength/lowest. atomic. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From science3.nasa.gov
Introduction to the Spectrum Science Mission Directorate Electromagnetic Spectrum Vs Atomic Emission Spectrum using a bohr model and the transition from n = 2 to n = 3 in an atom with a single electron, describe the mathematical relationship. Also, learn about the e mission spectrum and absorption spectrum. The unique pattern of light given off by an element when it is given energy; atomic emission spectroscopy (aes) is an analytical. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Electromagnetic Spectrum Vs Atomic Emission Spectrum atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the longest wavelength/lowest. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From hxewcajei.blob.core.windows.net
What Is Spectroscopy And Why Is It Important at Donald Fields blog Electromagnetic Spectrum Vs Atomic Emission Spectrum this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. To be in the lowest energy. emission spectrum (or atomic spectrum): Also, learn about the e mission spectrum and absorption spectrum. The unique pattern of light given off by an element when it is given energy; . Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From hxezpvsaw.blob.core.windows.net
Line Spectra Definition at Mayme Blackburn blog Electromagnetic Spectrum Vs Atomic Emission Spectrum atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. Also, learn about the e mission spectrum and absorption spectrum. this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. the shortest wavelength/highest energy light (violet 410. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From www.pinterest.es
emission spectra periodic table Google Search AP Chem 5 Atomic Electromagnetic Spectrum Vs Atomic Emission Spectrum the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the longest wavelength/lowest. atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. let us understand the phenomenon of dispersion of white light through a prism. To be in the lowest. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Electromagnetic Spectrum Vs Atomic Emission Spectrum atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. To be in the lowest energy. the shortest wavelength/highest energy light (violet. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From telegra.ph
Understanding the Spectrum Telegraph Electromagnetic Spectrum Vs Atomic Emission Spectrum emission spectrum (or atomic spectrum): atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. let us understand the phenomenon of. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From www.sciencelearn.org.nz
The spectrum — Science Learning Hub Electromagnetic Spectrum Vs Atomic Emission Spectrum The unique pattern of light given off by an element when it is given energy; using a bohr model and the transition from n = 2 to n = 3 in an atom with a single electron, describe the mathematical relationship. To be in the lowest energy. let us understand the phenomenon of dispersion of white light through. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From giofvqazo.blob.core.windows.net
What Is Spectroscopy Used For at Melissa Clayton blog Electromagnetic Spectrum Vs Atomic Emission Spectrum To be in the lowest energy. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. Also, learn about the e mission spectrum and absorption spectrum. let us understand the phenomenon of dispersion of white light through a prism. atomic emission. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From www.youtube.com
Spectrum Basic Introduction YouTube Electromagnetic Spectrum Vs Atomic Emission Spectrum atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. using a bohr model and the transition from n = 2 to. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From www.vrogue.co
The Same Concepts Used To Describe The Emission And A vrogue.co Electromagnetic Spectrum Vs Atomic Emission Spectrum this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. To be in the lowest energy. let us understand the phenomenon of dispersion of white light through a prism. the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From diagramopresivol1.z13.web.core.windows.net
Energy Diagram For Hydrogen Atom Electromagnetic Spectrum Vs Atomic Emission Spectrum atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. To be in the lowest energy. this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. let us understand the phenomenon of dispersion of white light through. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Electromagnetic Spectrum Vs Atomic Emission Spectrum this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the longest wavelength/lowest. To be in the lowest energy. let us understand the phenomenon of dispersion of white light. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From telegra.ph
Knowing the Spectrum involving Radiation Telegraph Electromagnetic Spectrum Vs Atomic Emission Spectrum To be in the lowest energy. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. let us understand the phenomenon of dispersion of white light through a prism. Also, learn about the e mission spectrum and absorption spectrum. using a. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Electromagnetic Spectrum Vs Atomic Emission Spectrum an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. let us understand the phenomenon of dispersion of white light through a prism. using a bohr model and the transition from n = 2 to n = 3 in an atom. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From mccord.cm.utexas.edu
Radiation Electromagnetic Spectrum Vs Atomic Emission Spectrum atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. To be in the lowest energy. using a bohr model and the transition from n = 2 to n = 3 in an atom with a single electron, describe the mathematical relationship. The unique pattern of light given off. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From mungfali.com
Hydrogen Atom Emission Spectrum Electromagnetic Spectrum Vs Atomic Emission Spectrum emission spectrum (or atomic spectrum): using a bohr model and the transition from n = 2 to n = 3 in an atom with a single electron, describe the mathematical relationship. The unique pattern of light given off by an element when it is given energy; the shortest wavelength/highest energy light (violet 410 nm) causes the electron. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From webmis.highland.cc.il.us
Atomic Spectra and Models of the Atom Electromagnetic Spectrum Vs Atomic Emission Spectrum this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. The unique pattern of light given off by an element when it is given energy; an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From byjus.com
Emission Spectrum Atomic Spectra Comparison Chemistry Byju's Electromagnetic Spectrum Vs Atomic Emission Spectrum Also, learn about the e mission spectrum and absorption spectrum. The unique pattern of light given off by an element when it is given energy; an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. let us understand the phenomenon of dispersion. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From telegra.ph
Understanding how to understand the Spectrum Telegraph Electromagnetic Spectrum Vs Atomic Emission Spectrum emission spectrum (or atomic spectrum): atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. using a bohr model and the transition from n = 2 to n = 3 in an atom with a single electron, describe the mathematical relationship. this module is designed to introduce. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From chem.libretexts.org
5.2 The Spectrum Chemistry LibreTexts Electromagnetic Spectrum Vs Atomic Emission Spectrum the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the longest wavelength/lowest. this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. using a bohr model and the transition from n = 2 to n = 3 in. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From telegra.ph
Exploring the Spectrum Telegraph Electromagnetic Spectrum Vs Atomic Emission Spectrum emission spectrum (or atomic spectrum): an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. the shortest wavelength/highest energy light (violet. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From dxoozpleb.blob.core.windows.net
And Spectrum at Katie Sullivan blog Electromagnetic Spectrum Vs Atomic Emission Spectrum atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. emission spectrum (or atomic spectrum): using a bohr model and the transition from n = 2 to n = 3 in an atom with a single electron, describe the mathematical relationship. the shortest wavelength/highest energy light (violet. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From www.epa.gov
Radiation Basics US EPA Electromagnetic Spectrum Vs Atomic Emission Spectrum atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. Also, learn about the e mission spectrum and absorption spectrum. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. let us. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From imagine.gsfc.nasa.gov
Spectrum Electromagnetic Spectrum Vs Atomic Emission Spectrum emission spectrum (or atomic spectrum): Also, learn about the e mission spectrum and absorption spectrum. the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the longest wavelength/lowest. The unique pattern of light given off by an element when it is given energy; let us understand the phenomenon of dispersion. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From pediaa.com
Difference Between Absorption and Emission Spectra Definition Electromagnetic Spectrum Vs Atomic Emission Spectrum let us understand the phenomenon of dispersion of white light through a prism. this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. The unique pattern of light given off by an element when it is given energy; emission spectrum (or atomic spectrum): an atomic. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From documents.pub
Spectrum Atomic Emission Spectrum. [PPTX Powerpoint] Electromagnetic Spectrum Vs Atomic Emission Spectrum Also, learn about the e mission spectrum and absorption spectrum. atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. using a bohr model and the transition. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From chemistrybook2011.blogspot.com
knowledge sea ATOMIC SPECTRUM Electromagnetic Spectrum Vs Atomic Emission Spectrum To be in the lowest energy. emission spectrum (or atomic spectrum): atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The unique pattern of light given off by an element when it is given energy; let us understand the phenomenon of dispersion of white light through a. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From studybreathings.z21.web.core.windows.net
How Is Visible Light Produced Electromagnetic Spectrum Vs Atomic Emission Spectrum The unique pattern of light given off by an element when it is given energy; let us understand the phenomenon of dispersion of white light through a prism. the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the longest wavelength/lowest. emission spectrum (or atomic spectrum): this module is. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From www.myxxgirl.com
What Is The Difference Between Emission Spectrum And Atomic Or Line Electromagnetic Spectrum Vs Atomic Emission Spectrum an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. emission spectrum (or atomic spectrum): Also, learn about the e mission spectrum and absorption spectrum. this module is designed to introduce the basic concepts of spectroscopy and to provide a survey. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From chem.libretexts.org
Chapter 2.3 Atomic Spectra and Models of the Atom Chemistry LibreTexts Electromagnetic Spectrum Vs Atomic Emission Spectrum let us understand the phenomenon of dispersion of white light through a prism. using a bohr model and the transition from n = 2 to n = 3 in an atom with a single electron, describe the mathematical relationship. the shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Electromagnetic Spectrum Vs Atomic Emission Spectrum Also, learn about the e mission spectrum and absorption spectrum. using a bohr model and the transition from n = 2 to n = 3 in an atom with a single electron, describe the mathematical relationship. atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. emission spectrum. Electromagnetic Spectrum Vs Atomic Emission Spectrum.
From www.sciencefriday.com
The Awesome Energy Of The Sun Science Friday Electromagnetic Spectrum Vs Atomic Emission Spectrum this module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. emission spectrum (or atomic spectrum): Also, learn about the e. Electromagnetic Spectrum Vs Atomic Emission Spectrum.