What Is Ideal Gas Molar Volume . ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,. the four gas variables are: the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). Using the ideal gas law and assuming standard. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. It is a good approximation of the. All of the empirical gas relationships are special. molar volume is defined as the volume occupied by one mole of a gas. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas.
from centraltutors.co.uk
It is a good approximation of the. the four gas variables are: use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. All of the empirical gas relationships are special. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. Using the ideal gas law and assuming standard.
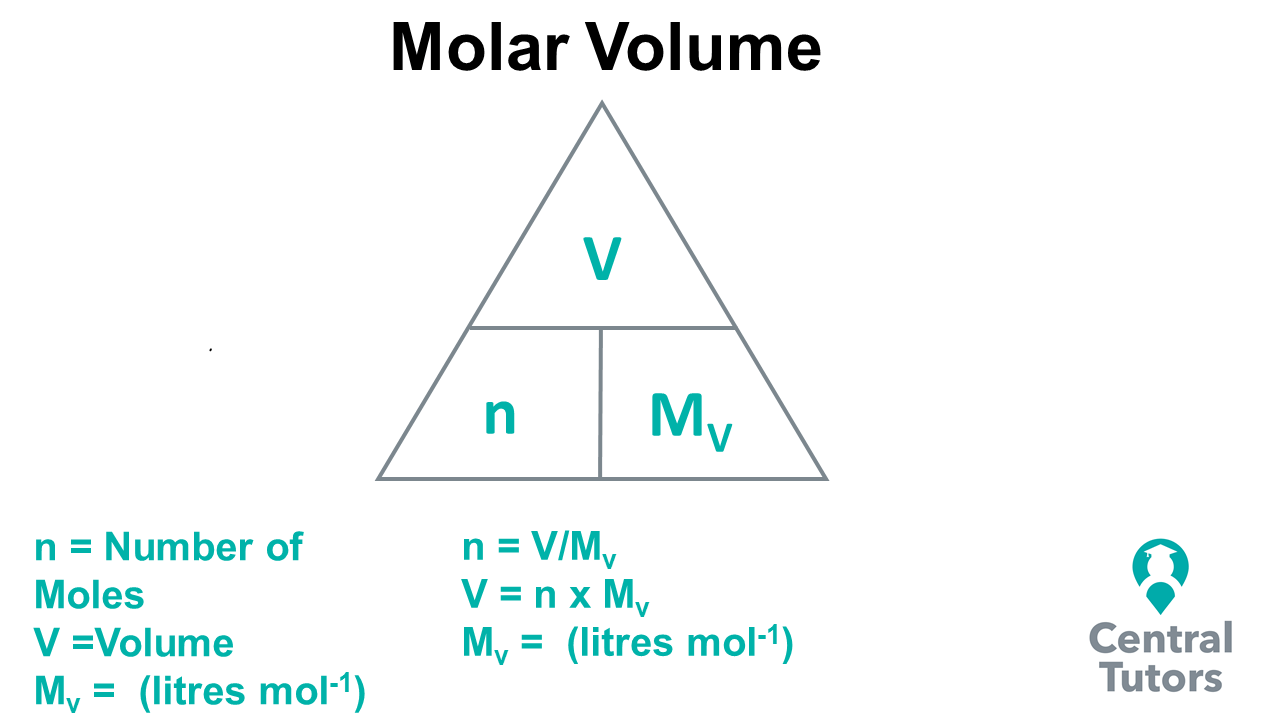
How to solve Mole Calculations using Molar Volume Central Tutors
What Is Ideal Gas Molar Volume use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. All of the empirical gas relationships are special. the four gas variables are: Using the ideal gas law and assuming standard. molar volume is defined as the volume occupied by one mole of a gas. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). It is a good approximation of the. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas.
From www.youtube.com
Calculate the Molar Mass of the Gas Ideal Gas Law with Molar Mass What Is Ideal Gas Molar Volume ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. It is a good approximation of the. All of the empirical gas relationships are special. . What Is Ideal Gas Molar Volume.
From www.slideserve.com
PPT Mole and gas volume PowerPoint Presentation, free download ID What Is Ideal Gas Molar Volume Using the ideal gas law and assuming standard. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). ideal gas law, relation between the pressure p, volume v, and temperature t of a. What Is Ideal Gas Molar Volume.
From www.slideserve.com
PPT Molar Volume and Ideal Gas Law PowerPoint Presentation, free What Is Ideal Gas Molar Volume It is a good approximation of the. the four gas variables are: the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,.. What Is Ideal Gas Molar Volume.
From www.chegg.com
Solved Which of the graphs below shows the relationship What Is Ideal Gas Molar Volume ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). molar volume is defined as the volume occupied by one mole of a gas. All of the empirical gas relationships. What Is Ideal Gas Molar Volume.
From www.youtube.com
Ideal Gas Equation Molar Volume at Standard Pressure and Temperature What Is Ideal Gas Molar Volume the four gas variables are: All of the empirical gas relationships are special. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. ideal gas law, relation between the pressure p, volume v, and. What Is Ideal Gas Molar Volume.
From www.youtube.com
5.5 Application of the Ideal Gas Law Molar Volumes, Density, & Molar What Is Ideal Gas Molar Volume the four gas variables are: molar volume is defined as the volume occupied by one mole of a gas. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature. What Is Ideal Gas Molar Volume.
From www.youtube.com
Molar Volume YouTube What Is Ideal Gas Molar Volume molar volume is defined as the volume occupied by one mole of a gas. the four gas variables are: ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. It is a good approximation of the. the ideal gas law, also called the general gas equation,. What Is Ideal Gas Molar Volume.
From www.youtube.com
14The mole and the volume of gases (1st year secondary first term What Is Ideal Gas Molar Volume Pressure (p), volume (v), number of mole of gas (n), and temperature (t). molar volume is defined as the volume occupied by one mole of a gas. Using the ideal gas law and assuming standard. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and. What Is Ideal Gas Molar Volume.
From www.slideserve.com
PPT Chapter 1 Gases PowerPoint Presentation, free download ID4564669 What Is Ideal Gas Molar Volume ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. It is a good approximation of the. the four gas variables are: the ideal. What Is Ideal Gas Molar Volume.
From mavink.com
How To Calculate Molar Volume Of A Gas What Is Ideal Gas Molar Volume All of the empirical gas relationships are special. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. Using the ideal gas law and assuming standard. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of. What Is Ideal Gas Molar Volume.
From www.youtube.com
Using the Ideal Gas Law to find density or molar mass YouTube What Is Ideal Gas Molar Volume Using the ideal gas law and assuming standard. the four gas variables are: use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. ideal. What Is Ideal Gas Molar Volume.
From www.chegg.com
Solved P 1 Consider an ideal gas with molar constant volume What Is Ideal Gas Molar Volume the four gas variables are: ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. Using the ideal gas law and assuming standard. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. All of the empirical gas relationships. What Is Ideal Gas Molar Volume.
From byjus.com
One mole of an ideal gas whose pressure changes with volume as P=αV What Is Ideal Gas Molar Volume Pressure (p), volume (v), number of mole of gas (n), and temperature (t). All of the empirical gas relationships are special. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in. What Is Ideal Gas Molar Volume.
From mmerevise.co.uk
The Ideal Gas Equation MME What Is Ideal Gas Molar Volume the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. molar volume is defined as the volume occupied by one mole of a gas. All of the empirical gas relationships are special. ideal gas law, relation between the pressure p, volume v, and temperature t of a. What Is Ideal Gas Molar Volume.
From www.sliderbase.com
The Ideal Gas Law Presentation Chemistry What Is Ideal Gas Molar Volume the four gas variables are: ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. Using the ideal gas law. What Is Ideal Gas Molar Volume.
From www.youtube.com
Molar Volume Calculated Two Different Ways YouTube What Is Ideal Gas Molar Volume the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or. What Is Ideal Gas Molar Volume.
From www.youtube.com
11.8 The Ideal Gas Law Pressure, Volume, Temperature, & Moles YouTube What Is Ideal Gas Molar Volume All of the empirical gas relationships are special. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,. molar volume is defined as the volume occupied by one mole of. What Is Ideal Gas Molar Volume.
From centraltutors.co.uk
How to solve Mole Calculations using Molar Volume Central Tutors What Is Ideal Gas Molar Volume ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. the ideal gas law, also called the general gas equation, is the equation of. What Is Ideal Gas Molar Volume.
From www.youtube.com
Molar Volume of a Gas Lab Part 1 YouTube What Is Ideal Gas Molar Volume the four gas variables are: molar volume is defined as the volume occupied by one mole of a gas. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit. What Is Ideal Gas Molar Volume.
From www.youtube.com
Volume of 1 mole of any gas at STP using the ideal gas equation YouTube What Is Ideal Gas Molar Volume ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,. molar volume is defined as the volume occupied by one mole of a gas. All of the empirical gas relationships are special. Pressure (p), volume (v), number of mole of gas (n), and. What Is Ideal Gas Molar Volume.
From www.youtube.com
Molar Volume of Gases The Mole Concept YouTube What Is Ideal Gas Molar Volume ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. the four gas variables are: molar volume is defined as the volume occupied by one mole of a gas. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). It is a good approximation. What Is Ideal Gas Molar Volume.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID What Is Ideal Gas Molar Volume the four gas variables are: molar volume is defined as the volume occupied by one mole of a gas. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,. Pressure (p), volume (v), number of mole of gas (n), and temperature (t).. What Is Ideal Gas Molar Volume.
From www.savemyexams.co.uk
Calculate Volumes of Gases (1.5.10) Edexcel IGCSE Chemistry Revision What Is Ideal Gas Molar Volume the four gas variables are: All of the empirical gas relationships are special. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. use the ideal. What Is Ideal Gas Molar Volume.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID6591194 What Is Ideal Gas Molar Volume It is a good approximation of the. the four gas variables are: All of the empirical gas relationships are special. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,. the volume of 1 mol of an ideal gas at stp is. What Is Ideal Gas Molar Volume.
From www.slideshare.net
Ideal gas law practice mccpot What Is Ideal Gas Molar Volume Using the ideal gas law and assuming standard. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in. What Is Ideal Gas Molar Volume.
From www.youtube.com
Q. 2.17 One mole of an ideal gas at standard temperature and pressure What Is Ideal Gas Molar Volume the four gas variables are: the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. use the ideal gas law to calculate pressure change, temperature change, volume change,. What Is Ideal Gas Molar Volume.
From www.youtube.com
CHEMISTRY 101 Density of a gas, molar volume, and molar mass YouTube What Is Ideal Gas Molar Volume ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. Using the ideal gas law and assuming standard. It is a good approximation of the. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and. What Is Ideal Gas Molar Volume.
From www.slideserve.com
PPT Molar Volume of a Gas PowerPoint Presentation, free download ID What Is Ideal Gas Molar Volume the four gas variables are: ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called. molar volume is defined as the volume occupied by one mole of a gas. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar. What Is Ideal Gas Molar Volume.
From chemistrymadesimple.net
Molar Volume of Gases What It Is and How To Use It Chemistry Made What Is Ideal Gas Molar Volume use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. All of the empirical gas. What Is Ideal Gas Molar Volume.
From www.alamy.com
Scientific Designing of The Mole And Molar Volume Formula Triangle What Is Ideal Gas Molar Volume the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. All of the empirical gas relationships are special. Using the ideal gas law and assuming standard. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. use the ideal. What Is Ideal Gas Molar Volume.
From www.toppr.com
An ideal gas has a molar heat capacity Cv at constant volume. Find the What Is Ideal Gas Molar Volume Pressure (p), volume (v), number of mole of gas (n), and temperature (t). It is a good approximation of the. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas. What Is Ideal Gas Molar Volume.
From www.slideserve.com
PPT Lesson 2 The Molar Relationships PowerPoint Presentation, free What Is Ideal Gas Molar Volume It is a good approximation of the. All of the empirical gas relationships are special. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. the four gas variables are: Using the ideal gas law and assuming standard. molar volume is defined as the volume occupied by one mole. What Is Ideal Gas Molar Volume.
From www.slideserve.com
PPT Molar Volume and Ideal Gas Law PowerPoint Presentation, free What Is Ideal Gas Molar Volume Pressure (p), volume (v), number of mole of gas (n), and temperature (t). use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. It is a good approximation of the. molar volume is defined as the volume occupied by one mole of a gas. ideal. What Is Ideal Gas Molar Volume.
From www.youtube.com
Applications of the Ideal Gas Law Molar Volume YouTube What Is Ideal Gas Molar Volume the four gas variables are: the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the. Using the ideal gas law and assuming standard. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of. What Is Ideal Gas Molar Volume.
From www.nagwa.com
Lesson Video Standard Molar Gas Volumes Nagwa What Is Ideal Gas Molar Volume use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in. What Is Ideal Gas Molar Volume.