Is Hf Strong Or Weak . Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones. Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. The terms strong and weak give an indication of the strength of an acid or base. There are only seven common strong acids, so one of the easiest ways to tell strong. However, it is a weak acid and not a strong acid because it does not. Define a strong and a weak acid and base. Hydrofluoric acid or hf is an extremely corrosive acid. A weak acid only partially dissociates in water to give h + and the anion. Recognize an acid or a base as strong or weak. Determine if a salt produces an acidic or.
from www.numerade.com
Determine if a salt produces an acidic or. However, it is a weak acid and not a strong acid because it does not. There are only seven common strong acids, so one of the easiest ways to tell strong. The terms strong and weak give an indication of the strength of an acid or base. Hydrofluoric acid or hf is an extremely corrosive acid. A weak acid only partially dissociates in water to give h + and the anion. Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones. Recognize an acid or a base as strong or weak. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh.
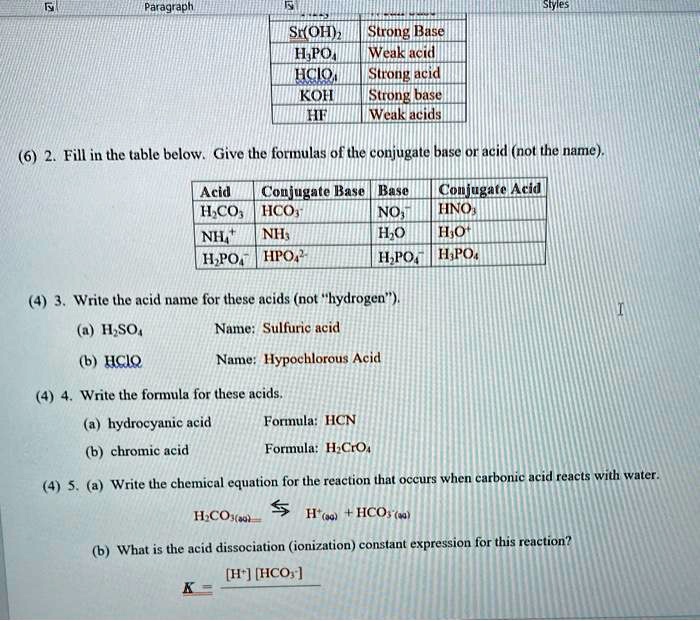
SOLVED Acid SrOH) Strong Base HPOA Weak Acid HClO Strong Acid KOH Strong Base HF Weak Acid
Is Hf Strong Or Weak The terms strong and weak give an indication of the strength of an acid or base. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. Define a strong and a weak acid and base. Determine if a salt produces an acidic or. There are only seven common strong acids, so one of the easiest ways to tell strong. The terms strong and weak give an indication of the strength of an acid or base. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. Recognize an acid or a base as strong or weak. Hydrofluoric acid or hf is an extremely corrosive acid. However, it is a weak acid and not a strong acid because it does not. Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones. A weak acid only partially dissociates in water to give h + and the anion.
From www.youtube.com
Why hydrogen fluoride is a weaker acid than hydrochloric acid Find acidic order of HF,HCl,HBr Is Hf Strong Or Weak The terms strong and weak give an indication of the strength of an acid or base. A weak acid only partially dissociates in water to give h + and the anion. Hydrofluoric acid or hf is an extremely corrosive acid. Define a strong and a weak acid and base. Recognize an acid or a base as strong or weak. There. Is Hf Strong Or Weak.
From www.slideshare.net
1 22 What Are Strong Acids And Bases Is Hf Strong Or Weak Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. There are only seven common strong acids, so one of the easiest ways to tell strong. A weak acid only partially dissociates in water to give h + and the anion. The terms strong and weak give an indication of the strength of an acid or. Is Hf Strong Or Weak.
From www.numerade.com
SOLVED Identify hydrofluoric acid strong electrolyte, strong acid weak electrolyte, strong acid Is Hf Strong Or Weak Determine if a salt produces an acidic or. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. Define a strong and a weak acid and base. Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. Hydrofluoric acid or hf is an extremely corrosive acid. Hydrofluoric acid is a. Is Hf Strong Or Weak.
From sciencenotes.org
Is Hydrofluoric Acid a Strong or Weak Acid? Is Hf Strong Or Weak However, it is a weak acid and not a strong acid because it does not. Define a strong and a weak acid and base. The terms strong and weak give an indication of the strength of an acid or base. Hydrofluoric acid or hf is an extremely corrosive acid. Examples of weak acids include hydrofluoric acid, hf, and acetic acid,. Is Hf Strong Or Weak.
From www.youtube.com
Why is hydrofluoric acid, HF, a weak acid compared to other hydrogen halides? YouTube Is Hf Strong Or Weak Define a strong and a weak acid and base. A weak acid only partially dissociates in water to give h + and the anion. However, it is a weak acid and not a strong acid because it does not. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability. Is Hf Strong Or Weak.
From www.quirkyscience.com
Hydrofluoric Acid A Weak Acid Yet It Dissolves Glass? Quirky Science Is Hf Strong Or Weak Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. The terms strong and weak give an. Is Hf Strong Or Weak.
From www.numerade.com
SOLVEDThe deciding factor on why HF is a weak acid and not a strong acid like the other Is Hf Strong Or Weak Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. There are only seven common strong acids, so one of the easiest ways to tell strong. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. A weak acid only. Is Hf Strong Or Weak.
From www.numerade.com
SOLVEDClassify each of the following species as a weak or strong acid (a) HNO3, (b) HF, (c) H2 Is Hf Strong Or Weak The terms strong and weak give an indication of the strength of an acid or base. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. A weak acid only partially dissociates in water to give h + and the anion. Hydrofluoric acid, while a weak. Is Hf Strong Or Weak.
From slideplayer.com
Which items do you think are ACIDS & which are BASES? ppt download Is Hf Strong Or Weak Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones. A weak acid only partially dissociates in water to give h + and the anion. There are only seven common strong acids, so one of the easiest ways to tell strong. Hydrofluoric acid is a weak acid compared to many others, but its health risks. Is Hf Strong Or Weak.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation ID6155898 Is Hf Strong Or Weak Hydrofluoric acid or hf is an extremely corrosive acid. Recognize an acid or a base as strong or weak. Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones. The terms strong and weak give an indication of the strength of an acid or base. A weak acid only partially dissociates in water to give. Is Hf Strong Or Weak.
From www.numerade.com
SOLVED acid strong or weak? species present at 106 mol/L or greater when dissolved in water Is Hf Strong Or Weak Hydrofluoric acid or hf is an extremely corrosive acid. A weak acid only partially dissociates in water to give h + and the anion. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch. Is Hf Strong Or Weak.
From www.slideserve.com
PPT Chapter 15 Acids and Bases PowerPoint Presentation, free download ID6594183 Is Hf Strong Or Weak Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones. However, it is a weak acid and not a strong acid because it does not. There are only seven common strong acids, so one of the easiest ways to tell strong. The terms strong and weak describe the ability of acid and base solutions to. Is Hf Strong Or Weak.
From answerhappy.com
A solution of the weak acid HF and a solution of the strong acid HCI have the same pH. Which Is Hf Strong Or Weak Define a strong and a weak acid and base. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. A weak acid only partially dissociates in water to give h + and the anion. However, it is a weak acid and not a strong acid because it does not. The terms strong and weak. Is Hf Strong Or Weak.
From topblogtenz.com
Is HF an acid or base? Strong or Weak Hydrogen fluoride Is Hf Strong Or Weak Recognize an acid or a base as strong or weak. Determine if a salt produces an acidic or. Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. There are only seven common strong acids, so one of the easiest ways to tell strong. Hydrofluoric acid, while a weak acid, would pass through your hand and. Is Hf Strong Or Weak.
From www.chegg.com
Solved Problem 9.29 4 of 5 > Constants Periodic Table HF Is Hf Strong Or Weak However, it is a weak acid and not a strong acid because it does not. There are only seven common strong acids, so one of the easiest ways to tell strong. Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. The terms strong and weak give an indication of the strength of an acid or. Is Hf Strong Or Weak.
From www.youtube.com
Is HF (Hydrofluoric acid) an Electrolyte or NonElectrolyte? YouTube Is Hf Strong Or Weak Hydrofluoric acid or hf is an extremely corrosive acid. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. There are only seven common strong acids, so one of the easiest ways to tell strong. The terms strong and weak describe the ability of acid and. Is Hf Strong Or Weak.
From www.thoughtco.com
Is Hydrofluoric Acid (HF) a Strong or Weak Acid? Is Hf Strong Or Weak A weak acid only partially dissociates in water to give h + and the anion. Determine if a salt produces an acidic or. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. The terms strong and weak describe the ability of acid and base solutions. Is Hf Strong Or Weak.
From www.chegg.com
Solved KF is a strong electrolyte, and HF is a weak Is Hf Strong Or Weak However, it is a weak acid and not a strong acid because it does not. The terms strong and weak give an indication of the strength of an acid or base. There are only seven common strong acids, so one of the easiest ways to tell strong. Hydrofluoric acid, while a weak acid, would pass through your hand and attack. Is Hf Strong Or Weak.
From www.numerade.com
SOLVED Classify each acid as strong or weak. (a) HCI (b) HF (c) HBr (d) H2 SO3 Numerade Is Hf Strong Or Weak Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. The terms strong and weak give an indication of the strength of an acid or base. Determine if a salt produces an acidic or. Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch. Is Hf Strong Or Weak.
From www.transtutors.com
(Solved) Strong Acid HF Weak Acid HI Strong Base NaClO4 Weak Base NaI... (1 Answer) Transtutors Is Hf Strong Or Weak Determine if a salt produces an acidic or. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. There are only seven common strong acids, so one of the easiest ways to tell strong. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its. Is Hf Strong Or Weak.
From www.numerade.com
SOLVEDExplain why HF is a weak acid, whereas HCl, HBr, and HI are all strong acids. Is Hf Strong Or Weak The terms strong and weak give an indication of the strength of an acid or base. There are only seven common strong acids, so one of the easiest ways to tell strong. Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. Hydrofluoric acid or hf is an extremely corrosive acid. A weak acid only partially. Is Hf Strong Or Weak.
From www.numerade.com
SOLVED Acid SrOH) Strong Base HPOA Weak Acid HClO Strong Acid KOH Strong Base HF Weak Acid Is Hf Strong Or Weak Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. Define a strong and a weak acid and base. The terms strong and weak give an indication of the strength of an acid or base. The terms strong and weak describe the ability of acid and. Is Hf Strong Or Weak.
From www.numerade.com
SOLVED HCI (aq) is a strong acid, which means that HCl is a stronger acid than H2O. HF and HCN Is Hf Strong Or Weak Determine if a salt produces an acidic or. Define a strong and a weak acid and base. There are only seven common strong acids, so one of the easiest ways to tell strong. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. A weak acid. Is Hf Strong Or Weak.
From www.chegg.com
Solved Which Diagram Represents An Aqueous Solution Of HF... Is Hf Strong Or Weak Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. Hydrofluoric acid or hf is an extremely corrosive acid. However, it is a weak acid and not a strong acid because it does not. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. The terms strong and weak give. Is Hf Strong Or Weak.
From www.vrogue.co
Hydrofluoric Acid Hf Is A Weak Acid With K 3 5 10 4 T vrogue.co Is Hf Strong Or Weak However, it is a weak acid and not a strong acid because it does not. There are only seven common strong acids, so one of the easiest ways to tell strong. Define a strong and a weak acid and base. Recognize an acid or a base as strong or weak. Determine if a salt produces an acidic or. Examples of. Is Hf Strong Or Weak.
From www.chegg.com
Solved Part A LiF is a strong electrolyte, and HF is a weak Is Hf Strong Or Weak Define a strong and a weak acid and base. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. There are only seven common strong acids, so one of the easiest ways to tell strong. Hydrofluoric acid or hf is an extremely corrosive acid. The terms. Is Hf Strong Or Weak.
From sciencenotes.org
Is Hydrofluoric Acid a Strong or Weak Acid? Is Hf Strong Or Weak Define a strong and a weak acid and base. Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. The terms strong and weak give an indication of the strength of an acid or base. However, it is a weak acid and not a strong acid because it does not. There are only seven common strong. Is Hf Strong Or Weak.
From www.numerade.com
Classify each of these soluble solutes as strong electrolyte, weak electrolyte, or Is Hf Strong Or Weak Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. Recognize an acid or a base as strong or weak. However, it is a weak acid and not a strong acid. Is Hf Strong Or Weak.
From www.cardionerds.com
STRONGHF Trial Cardionerds Twitter Journal Club Is Hf Strong Or Weak Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones. There are only seven common strong acids, so one of the easiest ways to tell strong. Determine if a salt produces. Is Hf Strong Or Weak.
From fphoto.photoshelter.com
science chemistry hydrofluoric acid Fundamental Photographs The Art of Science Is Hf Strong Or Weak Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones. Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. However, it is a weak acid and not a strong acid because it does not. The terms strong and weak describe the ability of acid and base solutions to conduct electricity.. Is Hf Strong Or Weak.
From www.thoughtco.com
Is Hydrofluoric Acid (HF) a Strong or Weak Acid? Is Hf Strong Or Weak There are only seven common strong acids, so one of the easiest ways to tell strong. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones. Determine if a salt produces an acidic or. However, it is a weak. Is Hf Strong Or Weak.
From www.numerade.com
SOLVED In the reaction between a weak acid, such as hydrofluoric acid, HF, and a strong base Is Hf Strong Or Weak Hydrofluoric acid or hf is an extremely corrosive acid. Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones. However, it is a weak acid and not a strong acid because it does not. Recognize an acid or a base as strong or weak. Hydrofluoric acid is a weak acid compared to many others, but. Is Hf Strong Or Weak.
From www.numerade.com
SOLVED TABLE 14.5 STRONG ELECTROLYTES AND WEAK ELECTROLYTES STRONG ELECTROLYTES WEAK Is Hf Strong Or Weak Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. Recognize an acid or a base as strong or weak. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. There are only seven common strong acids, so one of the easiest ways to tell strong. Hydrofluoric acid, while a. Is Hf Strong Or Weak.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID3515884 Is Hf Strong Or Weak Determine if a salt produces an acidic or. Recognize an acid or a base as strong or weak. A weak acid only partially dissociates in water to give h + and the anion. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. Hydrofluoric acid, while a weak acid, would pass through your hand. Is Hf Strong Or Weak.
From www.pearson.com
HF is a weak electrolyte and HBr is a strong electrolyte. Which o... Channels for Pearson+ Is Hf Strong Or Weak Examples of weak acids include hydrofluoric acid, hf, and acetic acid, ch 3 cooh. Recognize an acid or a base as strong or weak. A weak acid only partially dissociates in water to give h + and the anion. However, it is a weak acid and not a strong acid because it does not. The terms strong and weak describe. Is Hf Strong Or Weak.