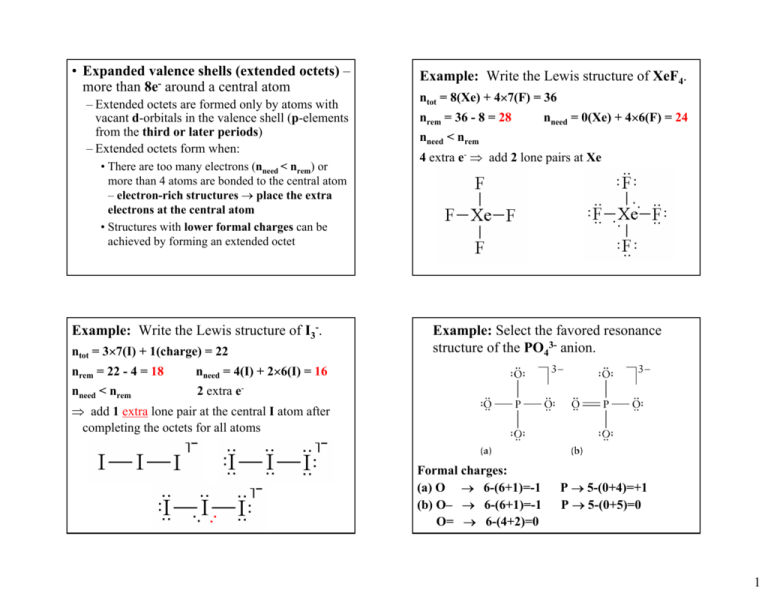
Example Of Expanded Valence Shell Molecules . One model to explain their existence uses one or more d orbitals in. For example, beryllium can form two covalent. there are three violations to the octet rule: an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. there are three violations to the octet rule: write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. the most common examples are the covalent compounds of beryllium and boron. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. molecules with expanded valence shells.
from studylib.net
an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. there are three violations to the octet rule: write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. One model to explain their existence uses one or more d orbitals in. For example, beryllium can form two covalent. the most common examples are the covalent compounds of beryllium and boron. molecules with expanded valence shells. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. there are three violations to the octet rule:
Expanded valence shells (extended octets)
Example Of Expanded Valence Shell Molecules the most common examples are the covalent compounds of beryllium and boron. write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. there are three violations to the octet rule: there are three violations to the octet rule: One model to explain their existence uses one or more d orbitals in. For example, beryllium can form two covalent. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. molecules with expanded valence shells. the most common examples are the covalent compounds of beryllium and boron.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. there are three violations to the octet rule: the most common examples are the covalent compounds of beryllium and boron. write the chemical formulas and chemical names. Example Of Expanded Valence Shell Molecules.
From www.slideserve.com
PPT Chemistry XL14A Chemical bonds PowerPoint Presentation, free download ID2313701 Example Of Expanded Valence Shell Molecules write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. One model to explain their existence uses one or more d orbitals in. molecules with expanded valence shells. In chemistry and. Example Of Expanded Valence Shell Molecules.
From www.youtube.com
Expanded Valence Shell Bonding YouTube Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. there are three violations to the octet rule: One model to explain their existence uses one or more d orbitals in. In chemistry and physics, an expanded octet is. Example Of Expanded Valence Shell Molecules.
From slidetodoc.com
Molecular Geometry Bonding Theories l Molecules have shapes Example Of Expanded Valence Shell Molecules molecules with expanded valence shells. the most common examples are the covalent compounds of beryllium and boron. One model to explain their existence uses one or more d orbitals in. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. there are three violations to the. Example Of Expanded Valence Shell Molecules.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules the most common examples are the covalent compounds of beryllium and boron. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. there are three violations to the octet rule: an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. write. Example Of Expanded Valence Shell Molecules.
From www.slideserve.com
PPT Lecture 24 Beyond the Octet PowerPoint Presentation, free download ID839011 Example Of Expanded Valence Shell Molecules an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. One model to explain their existence uses one or more d orbitals in. molecules with expanded valence shells. write the chemical formulas. Example Of Expanded Valence Shell Molecules.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules molecules with expanded valence shells. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. there are three violations to the octet rule: One model to explain their existence uses one or more d orbitals in. In chemistry and physics, an expanded octet is a molecule with. Example Of Expanded Valence Shell Molecules.
From www.slideserve.com
PPT Chemistry 100 Chapter 8 PowerPoint Presentation, free download ID2465420 Example Of Expanded Valence Shell Molecules write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. One model to explain their existence uses. Example Of Expanded Valence Shell Molecules.
From www.expii.com
Valence Electrons — Definition & Importance Expii Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: molecules with expanded valence shells. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. the most common examples are the covalent compounds of beryllium and boron. there are three violations to the octet rule: In chemistry. Example Of Expanded Valence Shell Molecules.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: For example, beryllium can form two covalent. there are three violations to the octet rule: write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. the most. Example Of Expanded Valence Shell Molecules.
From www.slideserve.com
PPT Molecular Geometry and Bonding Theories (CH.9) PowerPoint Presentation ID6128292 Example Of Expanded Valence Shell Molecules In chemistry and physics, an expanded octet is a molecule with an electron configuration that. molecules with expanded valence shells. write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. One model to explain their existence uses one or more d orbitals in. an atom like phosphorus or sulfur which has. Example Of Expanded Valence Shell Molecules.
From www.slideserve.com
PPT Molecular Shapes PowerPoint Presentation, free download ID6172622 Example Of Expanded Valence Shell Molecules an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. there are three violations to the octet rule: write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. In chemistry and physics, an expanded octet is a molecule with an electron. Example Of Expanded Valence Shell Molecules.
From www.youtube.com
Structural Chemistry I, Video IV Expanded Valence Shells YouTube Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: For example, beryllium can form two covalent. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. One model to explain their existence uses one or more d orbitals in. molecules with expanded valence shells. there are three violations to the octet rule: . Example Of Expanded Valence Shell Molecules.
From classnotes.org.in
Valence Shell Electron Pair Repulsion Theory Chemical Bonding and Molecular Structure Example Of Expanded Valence Shell Molecules For example, beryllium can form two covalent. there are three violations to the octet rule: an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. One model to explain their existence uses one or more d orbitals in. there are three violations to the octet rule: . Example Of Expanded Valence Shell Molecules.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules molecules with expanded valence shells. the most common examples are the covalent compounds of beryllium and boron. write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. there are. Example Of Expanded Valence Shell Molecules.
From slideplayer.com
Chapter 11 Chemical Bonding I Basic Concepts ppt download Example Of Expanded Valence Shell Molecules In chemistry and physics, an expanded octet is a molecule with an electron configuration that. write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. molecules with expanded valence shells. For example, beryllium can form two covalent. there are three violations to the octet rule: there are three violations to. Example Of Expanded Valence Shell Molecules.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: there are three violations to the octet rule: the most common examples are the covalent compounds of beryllium and boron. write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. One model to explain their existence uses one or more d orbitals. Example Of Expanded Valence Shell Molecules.
From www.slideserve.com
PPT Resonance Structures PowerPoint Presentation, free download ID5738499 Example Of Expanded Valence Shell Molecules molecules with expanded valence shells. write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. . Example Of Expanded Valence Shell Molecules.
From www.slideserve.com
PPT Lecture 24 Beyond the Octet PowerPoint Presentation, free download ID839011 Example Of Expanded Valence Shell Molecules For example, beryllium can form two covalent. write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. there are three violations to the octet rule: One model to explain their existence uses one or more d orbitals in. an atom like phosphorus or sulfur which has more than an octet is. Example Of Expanded Valence Shell Molecules.
From www.expii.com
Valence Electrons — Definition & Importance Expii Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: For example, beryllium can form two covalent. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. One model to explain their existence uses one. Example Of Expanded Valence Shell Molecules.
From www.slideserve.com
PPT Chapter 9 Molecular Geometries and Bonding Theories PowerPoint Presentation ID6519989 Example Of Expanded Valence Shell Molecules One model to explain their existence uses one or more d orbitals in. write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. the most common examples are the covalent compounds of beryllium and boron. molecules with expanded valence shells. For example, beryllium can form two covalent. there are three. Example Of Expanded Valence Shell Molecules.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules the most common examples are the covalent compounds of beryllium and boron. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. molecules with expanded valence shells. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. write the chemical formulas. Example Of Expanded Valence Shell Molecules.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules One model to explain their existence uses one or more d orbitals in. For example, beryllium can form two covalent. write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. there are three violations to the octet rule: In chemistry and physics, an expanded octet is a molecule with an electron configuration. Example Of Expanded Valence Shell Molecules.
From www.youtube.com
VSEPR for Expanded Valence Shell YouTube Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: molecules with expanded valence shells. For example, beryllium can form two covalent. the most common examples are the covalent compounds of beryllium and boron. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. One model to explain. Example Of Expanded Valence Shell Molecules.
From www.youtube.com
Grade12 Chapter1 Lewis Structures of Electron deficient and Expanded valence shell molecules Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: One model to explain their existence uses one or more d orbitals in. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. the most common examples are the covalent compounds of beryllium and boron. molecules with expanded valence shells. there are three. Example Of Expanded Valence Shell Molecules.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules In chemistry and physics, an expanded octet is a molecule with an electron configuration that. there are three violations to the octet rule: an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. the most common examples are the covalent compounds of beryllium and boron. For example,. Example Of Expanded Valence Shell Molecules.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Example Of Expanded Valence Shell Molecules In chemistry and physics, an expanded octet is a molecule with an electron configuration that. the most common examples are the covalent compounds of beryllium and boron. One model to explain their existence uses one or more d orbitals in. For example, beryllium can form two covalent. an atom like phosphorus or sulfur which has more than an. Example Of Expanded Valence Shell Molecules.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules molecules with expanded valence shells. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. One model to explain their existence uses one or more d orbitals in. there are three violations. Example Of Expanded Valence Shell Molecules.
From www.slideserve.com
PPT PowerPoint Presentation, free download ID2453814 Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: One model to explain their existence uses one or more d orbitals in. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. For example,. Example Of Expanded Valence Shell Molecules.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: In chemistry and physics, an expanded octet is a molecule with an electron configuration that. there are three violations to the octet rule: One model to explain their existence uses one or more d orbitals in. molecules with expanded valence shells. the most common examples are the covalent. Example Of Expanded Valence Shell Molecules.
From studylib.net
Expanded valence shells (extended octets) Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: One model to explain their existence uses one or more d orbitals in. molecules with expanded valence shells. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. the most common examples are the covalent compounds of beryllium and boron. there are three. Example Of Expanded Valence Shell Molecules.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules molecules with expanded valence shells. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. For example, beryllium can form two covalent. there are three violations to the octet rule: write. Example Of Expanded Valence Shell Molecules.
From www.slideserve.com
PPT Molecular Geometry PowerPoint Presentation ID2422169 Example Of Expanded Valence Shell Molecules there are three violations to the octet rule: write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. molecules with expanded valence shells. For example, beryllium can form two covalent. the most common examples are the covalent compounds of beryllium and boron. In chemistry and physics, an expanded octet is. Example Of Expanded Valence Shell Molecules.
From ar.inspiredpencil.com
Valence Electron Structure Example Of Expanded Valence Shell Molecules the most common examples are the covalent compounds of beryllium and boron. For example, beryllium can form two covalent. In chemistry and physics, an expanded octet is a molecule with an electron configuration that. molecules with expanded valence shells. there are three violations to the octet rule: there are three violations to the octet rule: . Example Of Expanded Valence Shell Molecules.
From www.sliderbase.com
Molecules with Expanded Valence Shells Example Of Expanded Valence Shell Molecules For example, beryllium can form two covalent. the most common examples are the covalent compounds of beryllium and boron. write the chemical formulas and chemical names of covalent molecules that contain atoms with expanded valences. there are three violations to the octet rule: In chemistry and physics, an expanded octet is a molecule with an electron configuration. Example Of Expanded Valence Shell Molecules.