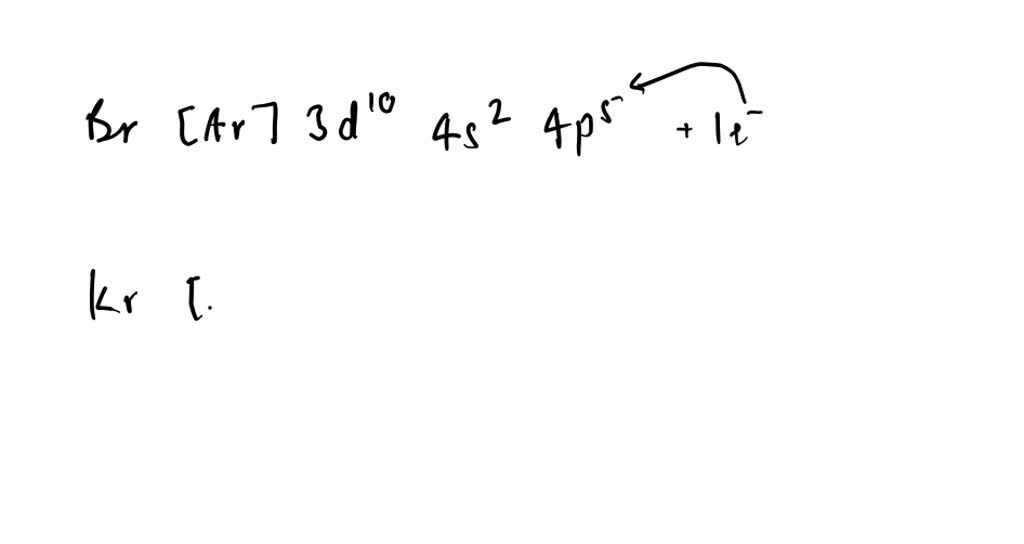Br Electron Affinity . This process differs from electronegativity, which we define as the ability of an atom to attract an electron toward itself. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. electron affinity of bromine. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. This process creates a negative ion. Similarly, use the trends in electron affinities from left to right for elements in the. Chemists define electron affinity as the change in energy, measured in units of kj/mole, experienced when an electron is added to a gaseous atom. The amount of energy released when an electron is added to atom) for most of. what is electron affinity? the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. use the trends in electron affinities going down a column for elements in the same group. In chemistry and atomic physics, the electron. So the more negative the. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. 103 rows table shows electron affinity (i.e.
from www.numerade.com
electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. Similarly, use the trends in electron affinities from left to right for elements in the. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. The amount of energy released when an electron is added to atom) for most of. This process creates a negative ion. use the trends in electron affinities going down a column for elements in the same group. So the more negative the. 103 rows table shows electron affinity (i.e. This process differs from electronegativity, which we define as the ability of an atom to attract an electron toward itself. In chemistry and atomic physics, the electron.
SOLVEDAlthough the electron affinity of bromine is a negative quantity
Br Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. 103 rows table shows electron affinity (i.e. use the trends in electron affinities going down a column for elements in the same group. Chemists define electron affinity as the change in energy, measured in units of kj/mole, experienced when an electron is added to a gaseous atom. In chemistry and atomic physics, the electron. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. Electron affinity of bromine is 324.6 kj/mol. This process creates a negative ion. This process differs from electronegativity, which we define as the ability of an atom to attract an electron toward itself. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. what is electron affinity? The amount of energy released when an electron is added to atom) for most of. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. electron affinity of bromine. Similarly, use the trends in electron affinities from left to right for elements in the.
From www.numerade.com
SOLVED Arrange these elements according to electron affinity Most Br Electron Affinity So the more negative the. use the trends in electron affinities going down a column for elements in the same group. Similarly, use the trends in electron affinities from left to right for elements in the. Electron affinity of bromine is 324.6 kj/mol. the electron affinity is the potential energy change of the atom when an electron is. Br Electron Affinity.
From www.slideserve.com
PPT CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES OF Br Electron Affinity Chemists define electron affinity as the change in energy, measured in units of kj/mole, experienced when an electron is added to a gaseous atom. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. The amount of energy released when an electron is added to atom). Br Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Br Electron Affinity what is electron affinity? 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. electron affinity of bromine. Chemists define electron affinity as the change in energy, measured in units of kj/mole, experienced when an electron is added to a gaseous atom. the electron affinity. Br Electron Affinity.
From pediabay.com
Electron Affinity Chart of Elements (With Periodic Table) Pediabay Br Electron Affinity Similarly, use the trends in electron affinities from left to right for elements in the. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. So the more negative the. In chemistry and atomic physics, the electron. the electron affinity is the potential energy change. Br Electron Affinity.
From www.breakingatom.com
Electron Affinity of The Elements Br Electron Affinity Electron affinity of bromine is 324.6 kj/mol. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. use the trends in electron affinities going down a column for elements in the same group. electron affinity of bromine. This process creates a negative ion. Chemists. Br Electron Affinity.
From www.youtube.com
Electron Affinity Equations YouTube Br Electron Affinity 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. what is electron affinity? 103 rows table shows electron affinity (i.e. This process creates a negative ion. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous. Br Electron Affinity.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Br Electron Affinity 103 rows table shows electron affinity (i.e. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. So the more negative the. The amount of energy released when an electron is added to atom) for most of. In chemistry and atomic physics, the electron. . Br Electron Affinity.
From www.youtube.com
069 Electron Affinity YouTube Br Electron Affinity the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. Chemists define electron affinity as the change in energy, measured in. Br Electron Affinity.
From www.numerade.com
SOLVEDAlthough the electron affinity of bromine is a negative quantity Br Electron Affinity Electron affinity of bromine is 324.6 kj/mol. Chemists define electron affinity as the change in energy, measured in units of kj/mole, experienced when an electron is added to a gaseous atom. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. So the more negative the. use. Br Electron Affinity.
From www.coursehero.com
[Solved] Use this electron affinity, EA, data for Cl, Br, and I (and Br Electron Affinity what is electron affinity? This process differs from electronegativity, which we define as the ability of an atom to attract an electron toward itself. Similarly, use the trends in electron affinities from left to right for elements in the. 103 rows table shows electron affinity (i.e. electron affinity is defined as the change in energy (in kj/mole). Br Electron Affinity.
From www.numerade.com
SOLVEDArrange the elements in each of the following groups in Br Electron Affinity the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. This process differs from electronegativity, which we define as the ability of an atom to attract an electron toward itself. The amount of energy released when an electron is added to atom) for. Br Electron Affinity.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Br Electron Affinity 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. The amount of energy released when an electron is added to atom) for most of. So the more negative the. In chemistry and atomic physics, the electron. electron affinity of bromine. 125 rows the electron affinity. Br Electron Affinity.
From www.numerade.com
SOLVEDWhich has the highest electron affinity Li or cs? Li or F Cs or Br Electron Affinity use the trends in electron affinities going down a column for elements in the same group. This process differs from electronegativity, which we define as the ability of an atom to attract an electron toward itself. Chemists define electron affinity as the change in energy, measured in units of kj/mole, experienced when an electron is added to a gaseous. Br Electron Affinity.
From exorwrmka.blob.core.windows.net
Bromine Of Electron Affinity at Curtis Phillips blog Br Electron Affinity 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. use the trends in electron affinities going down a column for elements in the same group. the electron affinity is the potential energy change of the atom when an electron is added to a. Br Electron Affinity.
From www.schoolmykids.com
Compare Bromine vs Iodine Periodic Table Element Comparison Compare Br Electron Affinity Similarly, use the trends in electron affinities from left to right for elements in the. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. This process differs from electronegativity, which we define as the ability of an atom to attract an electron toward itself. 103 rows. Br Electron Affinity.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Br Electron Affinity 103 rows table shows electron affinity (i.e. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. use the trends in electron affinities going down a column for elements in the same group. In chemistry and atomic physics, the electron. So. Br Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Br Electron Affinity Electron affinity of bromine is 324.6 kj/mol. Chemists define electron affinity as the change in energy, measured in units of kj/mole, experienced when an electron is added to a gaseous atom. This process differs from electronegativity, which we define as the ability of an atom to attract an electron toward itself. Similarly, use the trends in electron affinities from left. Br Electron Affinity.
From www.pinterest.com
Electron Affinity Electron affinity, Periodic table, Chemistry for kids Br Electron Affinity This process creates a negative ion. Electron affinity of bromine is 324.6 kj/mol. Chemists define electron affinity as the change in energy, measured in units of kj/mole, experienced when an electron is added to a gaseous atom. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron. Br Electron Affinity.
From material-properties.org
Bromine Periodic Table and Atomic Properties Br Electron Affinity The amount of energy released when an electron is added to atom) for most of. Similarly, use the trends in electron affinities from left to right for elements in the. This process creates a negative ion. Electron affinity of bromine is 324.6 kj/mol. use the trends in electron affinities going down a column for elements in the same group.. Br Electron Affinity.
From slideplayer.com
The Periodic Law (Periodic Table) ppt download Br Electron Affinity In chemistry and atomic physics, the electron. use the trends in electron affinities going down a column for elements in the same group. what is electron affinity? Similarly, use the trends in electron affinities from left to right for elements in the. Chemists define electron affinity as the change in energy, measured in units of kj/mole, experienced when. Br Electron Affinity.
From www.sliderbase.com
Periodic Behavior Presentation Chemistry Br Electron Affinity This process creates a negative ion. Similarly, use the trends in electron affinities from left to right for elements in the. electron affinity of bromine. So the more negative the. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. what is electron affinity?. Br Electron Affinity.
From periodictableguide.com
Electron Affinity Chart (Labeled Periodic table + List) Br Electron Affinity the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. 103 rows table shows electron affinity (i.e. Similarly, use the trends in electron affinities from left to right for elements in the. 125 rows the electron affinity is defined as the. Br Electron Affinity.
From www.pearson.com
The periodic table with electron affinity values is shown below Br Electron Affinity Electron affinity of bromine is 324.6 kj/mol. use the trends in electron affinities going down a column for elements in the same group. Similarly, use the trends in electron affinities from left to right for elements in the. This process differs from electronegativity, which we define as the ability of an atom to attract an electron toward itself. . Br Electron Affinity.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Br Electron Affinity electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. electron affinity of bromine. 103 rows table shows electron affinity (i.e. use the trends in electron affinities going down a column for elements in the same group. This process creates a negative ion.. Br Electron Affinity.
From www.toppr.com
The electron affinity of bromine atom is equal to the Br Electron Affinity 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. This process creates a negative ion. electron affinity of bromine. Electron affinity of bromine is 324.6 kj/mol. The amount of energy released when an electron is added to atom) for most of. In chemistry and atomic physics,. Br Electron Affinity.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Br Electron Affinity electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. So the more negative the. 103 rows table shows electron affinity (i.e. This process creates a negative ion. The amount of energy released when an electron is added to atom) for most of. Similarly, use. Br Electron Affinity.
From pubchem.ncbi.nlm.nih.gov
Electron Affinity Periodic Table of Elements PubChem Br Electron Affinity electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. Electron affinity of bromine is 324.6 kj/mol. Similarly, use the trends in electron affinities from left to right for elements in the. 103 rows table shows electron affinity (i.e. This process differs from electronegativity, which. Br Electron Affinity.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Br Electron Affinity electron affinity of bromine. use the trends in electron affinities going down a column for elements in the same group. In chemistry and atomic physics, the electron. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. The amount of energy released when an electron is. Br Electron Affinity.
From games.assurances.gov.gh
Br Electron Configuration Long Form Br Electron Affinity Electron affinity of bromine is 324.6 kj/mol. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. what is electron affinity? use the trends in electron affinities going down a column for elements in the same group. Chemists define electron affinity as the change in energy,. Br Electron Affinity.
From www.chegg.com
Solved Use this electron affinity, EA, data for Cl, Br, and Br Electron Affinity The amount of energy released when an electron is added to atom) for most of. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. electron affinity of bromine. 103 rows table shows electron affinity (i.e. use the trends in electron affinities going down a. Br Electron Affinity.
From sciencenotes.org
Electron Affinity Trend and Definition Br Electron Affinity electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is. In chemistry and atomic physics, the electron. The amount of energy released when an electron is added to atom) for most of. Similarly, use the trends in electron affinities from left to right for elements in. Br Electron Affinity.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Br Electron Affinity Chemists define electron affinity as the change in energy, measured in units of kj/mole, experienced when an electron is added to a gaseous atom. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. In chemistry and atomic physics, the electron. what is electron affinity? So the. Br Electron Affinity.
From www.youtube.com
Electron Affinity Trend, Basic Introduction, Chemistry YouTube Br Electron Affinity In chemistry and atomic physics, the electron. This process creates a negative ion. use the trends in electron affinities going down a column for elements in the same group. Similarly, use the trends in electron affinities from left to right for elements in the. what is electron affinity? This process differs from electronegativity, which we define as the. Br Electron Affinity.
From chem.libretexts.org
1.1.2.4 Electron Affinity Chemistry LibreTexts Br Electron Affinity Similarly, use the trends in electron affinities from left to right for elements in the. Electron affinity of bromine is 324.6 kj/mol. 103 rows table shows electron affinity (i.e. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. So the more negative the. . Br Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Br Electron Affinity The amount of energy released when an electron is added to atom) for most of. This process creates a negative ion. what is electron affinity? 103 rows table shows electron affinity (i.e. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. Similarly, use. Br Electron Affinity.