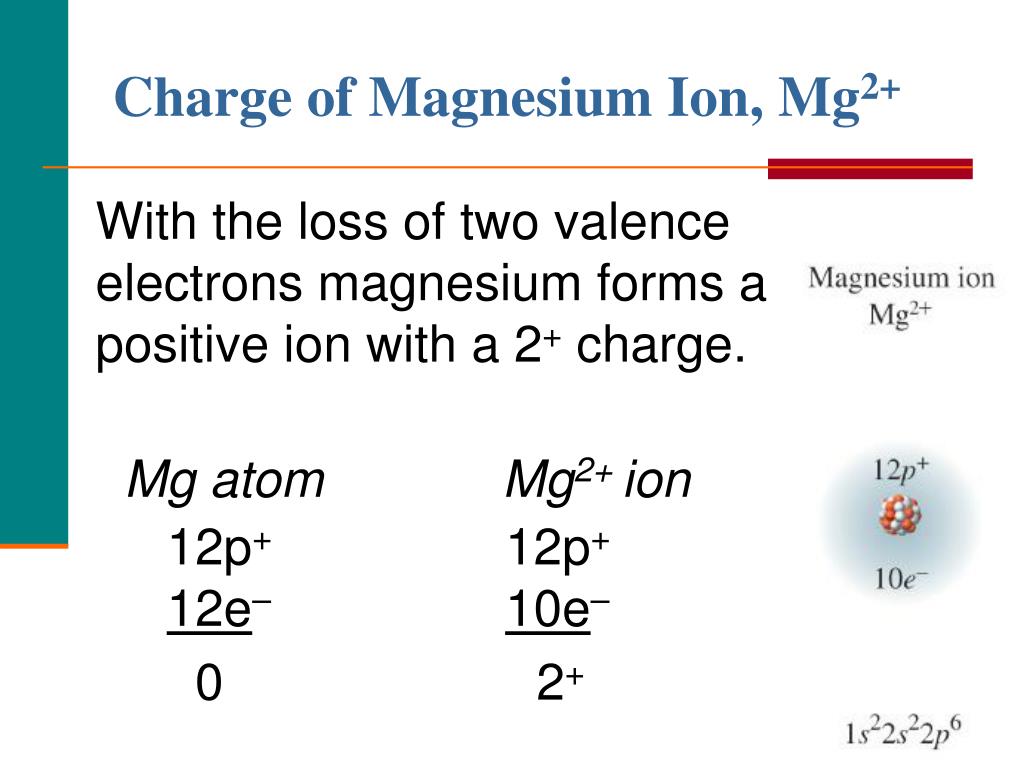
Magnesium Valence Electrons Ion . the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. The ground state electron configuration of magnesium is important. magnesium has two valence electrons in the 3s orbital. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,.
from www.slideserve.com
magnesium has two valence electrons in the 3s orbital. a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. The ground state electron configuration of magnesium is important. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,.
PPT The Octet Rule PowerPoint Presentation, free download ID2654700
Magnesium Valence Electrons Ion when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. magnesium has two valence electrons in the 3s orbital. these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. The ground state electron configuration of magnesium is important. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a.
From www.mikrora.com
File Electron Configuration Magnesium Svg Best Diagram Collection Magnesium Valence Electrons Ion a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. 93 rows. Magnesium Valence Electrons Ion.
From www.sciencephoto.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Library Magnesium Valence Electrons Ion the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. The ground state electron configuration of magnesium is important. a metal from group. Magnesium Valence Electrons Ion.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Valence Electrons Ion these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. The ground state electron configuration of magnesium is important. the electron arrangement also shows the number of valence electrons which is two for magnesium because. Magnesium Valence Electrons Ion.
From www.schoolmouv.fr
Représentation de Lewis d'un atome et d'une molécule cours 1ere Magnesium Valence Electrons Ion magnesium has two valence electrons in the 3s orbital. The ground state electron configuration of magnesium is important. these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the electron arrangement also shows the. Magnesium Valence Electrons Ion.
From www.slideshare.net
The periodic table Magnesium Valence Electrons Ion these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level.. Magnesium Valence Electrons Ion.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Magnesium Valence Electrons Ion when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,.. Magnesium Valence Electrons Ion.
From www.slideserve.com
PPT How many valence electrons does magnesium have? PowerPoint Magnesium Valence Electrons Ion these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. magnesium has two valence electrons in the 3s orbital. a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. when forming ions, elements typically gain or lose the minimum number of. Magnesium Valence Electrons Ion.
From periodictable.me
Magnesium Valence Electron Magnesium Valency (Mg) with Dot Diagram Magnesium Valence Electrons Ion a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. magnesium has two valence electrons in the 3s orbital. The ground state electron configuration of magnesium. Magnesium Valence Electrons Ion.
From www.slideserve.com
PPT Valence Electrons PowerPoint Presentation, free download ID651377 Magnesium Valence Electrons Ion the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. The. Magnesium Valence Electrons Ion.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Valence Electrons Ion The ground state electron configuration of magnesium is important. a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. when forming ions, elements typically gain or lose. Magnesium Valence Electrons Ion.
From cejxrkef.blob.core.windows.net
Magnesium Ion Nuclear Notation at Catherine Lackey blog Magnesium Valence Electrons Ion The ground state electron configuration of magnesium is important. a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. when forming ions, elements typically gain or lose. Magnesium Valence Electrons Ion.
From valenceelectrons.com
How to Find the Valence Electrons for Magnesium (Mg)? Magnesium Valence Electrons Ion a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. The ground state electron configuration of magnesium is important. magnesium. Magnesium Valence Electrons Ion.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Magnesium Valence Electrons Ion The ground state electron configuration of magnesium is important. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. these forms of magnesium. Magnesium Valence Electrons Ion.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Magnesium Valence Electrons Ion a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. The ground state electron configuration of magnesium is important. magnesium has two valence electrons in the 3s orbital. the electron arrangement also shows the number of valence electrons which is two for magnesium because there. Magnesium Valence Electrons Ion.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Valence Electrons Ion magnesium has two valence electrons in the 3s orbital. a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. The ground state electron configuration of magnesium is important. the electron arrangement also shows the number of valence electrons which is two for magnesium because there. Magnesium Valence Electrons Ion.
From mungfali.com
Magnesium Ion Electron Configuration Magnesium Valence Electrons Ion the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. The ground state electron configuration of magnesium is important. a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. magnesium. Magnesium Valence Electrons Ion.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Valence Electrons Ion the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. The ground state electron configuration of magnesium is important. these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. magnesium has two valence electrons in the 3s orbital. . Magnesium Valence Electrons Ion.
From valenceelectrons.com
Protons, Neutrons, Electrons for Magnesium (Mg, Mg2+) Magnesium Valence Electrons Ion the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. magnesium has two valence electrons in the 3s orbital. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. The ground state electron. Magnesium Valence Electrons Ion.
From www.youtube.com
Valence Electrons for Magnesium (Mg) YouTube Magnesium Valence Electrons Ion a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. magnesium has two valence electrons in the 3s orbital. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. . Magnesium Valence Electrons Ion.
From mydiagram.online
[DIAGRAM] Lewis Electron Dot Diagrams Magnesium Magnesium Valence Electrons Ion a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. magnesium has two valence electrons in the 3s orbital. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. . Magnesium Valence Electrons Ion.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Magnesium Valence Electrons Ion The ground state electron configuration of magnesium is important. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. magnesium has two valence electrons. Magnesium Valence Electrons Ion.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Valence Electrons Ion The ground state electron configuration of magnesium is important. these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the electron arrangement also shows the number of valence electrons which is two for magnesium because. Magnesium Valence Electrons Ion.
From dxoxehbxk.blob.core.windows.net
Magnesium Level Electrons at Robert Woods blog Magnesium Valence Electrons Ion the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. magnesium has two valence electrons in the 3s orbital. . Magnesium Valence Electrons Ion.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Magnesium Valence Electrons Ion magnesium has two valence electrons in the 3s orbital. The ground state electron configuration of magnesium is important. these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. when forming ions, elements typically gain or. Magnesium Valence Electrons Ion.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Valence Electrons Ion these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the. Magnesium Valence Electrons Ion.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Magnesium Valence Electrons Ion when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. a metal from group 2 (e.g.,. Magnesium Valence Electrons Ion.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Magnesium Valence Electrons Ion a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. The ground state electron configuration of magnesium is important. magnesium has two valence electrons in the 3s orbital. the electron arrangement also shows the number of valence electrons which is two for magnesium because there. Magnesium Valence Electrons Ion.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Valence Electrons Ion these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. magnesium has two valence electrons in the 3s orbital. a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. 93 rows this table of element valences includes the maximum valence and. Magnesium Valence Electrons Ion.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Valence Electrons Ion these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. . Magnesium Valence Electrons Ion.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Magnesium Valence Electrons Ion 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the electron arrangement also shows the. Magnesium Valence Electrons Ion.
From ceieufog.blob.core.windows.net
Magnesium Electron Configuration Ground State at Mae Santos blog Magnesium Valence Electrons Ion The ground state electron configuration of magnesium is important. magnesium has two valence electrons in the 3s orbital. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. when forming ions, elements typically gain or lose the minimum number of electrons necessary. Magnesium Valence Electrons Ion.
From periodictable.me
Magnesium Valence Electrons Archives Dynamic Periodic Table of Magnesium Valence Electrons Ion the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. magnesium has two valence electrons in the 3s orbital. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. a metal from. Magnesium Valence Electrons Ion.
From valenceelectrons.com
Electron Configuration for Magnesium and ion(Mg2+) Magnesium Valence Electrons Ion 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons. Magnesium Valence Electrons Ion.
From www.slideserve.com
PPT Valence Electrons and Oxidation Numbers PowerPoint Presentation Magnesium Valence Electrons Ion these forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride,. a metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a. magnesium has two valence electrons in the 3s orbital. when forming ions, elements typically gain or lose the minimum number of. Magnesium Valence Electrons Ion.
From ceieufog.blob.core.windows.net
Magnesium Electron Configuration Ground State at Mae Santos blog Magnesium Valence Electrons Ion 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. the electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the \(n=3\) energy level. when forming ions, elements typically gain or lose the minimum number of electrons necessary. Magnesium Valence Electrons Ion.