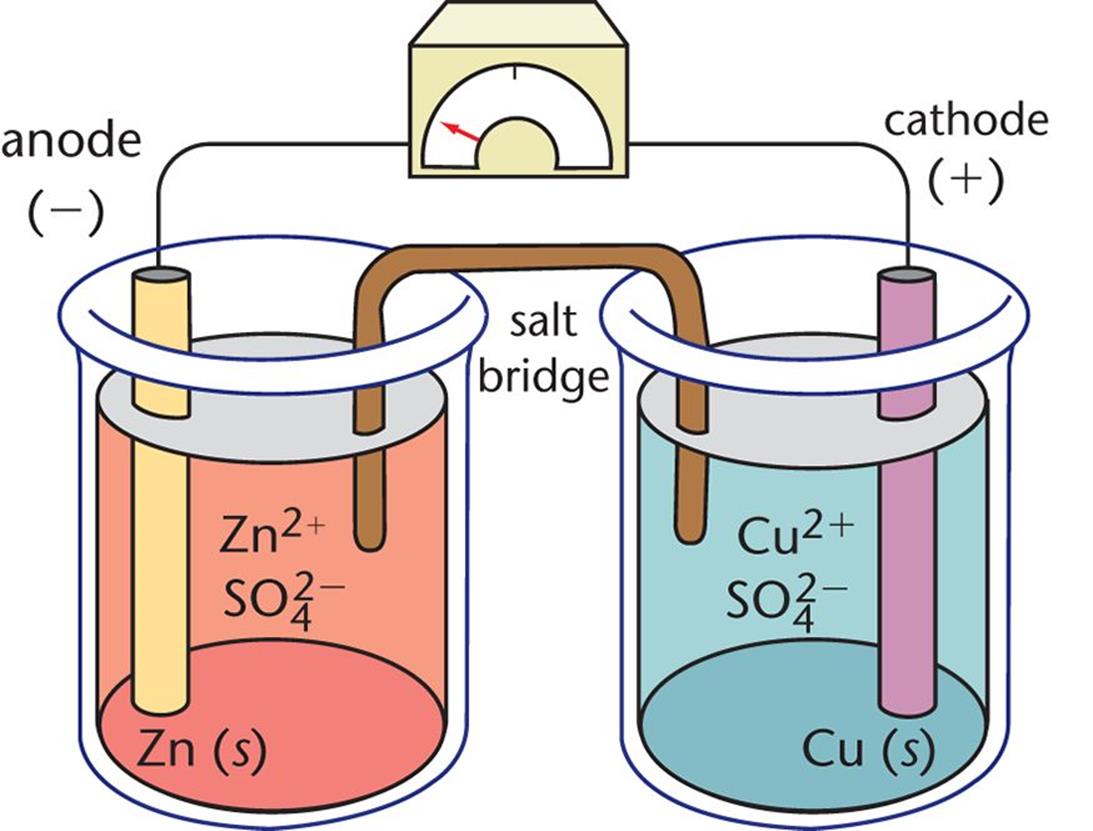
Electrodes Of Electrolytes . To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Electrode a conductor used to establish electrical contact with a circuit. Electrodes are vital components of electrochemical cells. The positive electrode, on the other hand, will attract negative ions (anions). Ionic compounds conduct electricity when molten or in solution. If we place a variable resistance. Ionic compounds conduct electricity when molten or in. Reactive metals are extracted from their ores using electrolysis. The electrode attached to the negative terminal of a battery is. Electrochemical cells allow measurement and control of a redox reaction. This charge is based off. Electrolyte is the ionic compound in a molten or dissolved solution that conducts. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Reactive metals are extracted from their ores using electrolysis. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode.
from schoolbag.info
Electrode a conductor used to establish electrical contact with a circuit. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Ionic compounds conduct electricity when molten or in. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. The reaction can be started and stopped by connecting or disconnecting the two electrodes. This charge is based off. The electrode attached to the negative terminal of a battery is. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive electrode, on the other hand, will attract negative ions (anions). Ionic compounds conduct electricity when molten or in solution.
Electrochemical Cells Electrochemistry Training MCAT General
Electrodes Of Electrolytes This charge is based off. This charge is based off. Ionic compounds conduct electricity when molten or in. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. The electrode attached to the negative terminal of a battery is. Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. Electrodes are vital components of electrochemical cells. Electrolyte is the ionic compound in a molten or dissolved solution that conducts. The positive electrode, on the other hand, will attract negative ions (anions). Electrode a conductor used to establish electrical contact with a circuit. The reaction can be started and stopped by connecting or disconnecting the two electrodes. If we place a variable resistance. Electrochemical cells allow measurement and control of a redox reaction.
From www.researchgate.net
Schematic view of the different electrodes and electrolytes for SCs Electrodes Of Electrolytes The positive electrode, on the other hand, will attract negative ions (anions). Ionic compounds conduct electricity when molten or in solution. This charge is based off. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Electrodes are vital components of electrochemical cells. Electrode a conductor used to establish electrical. Electrodes Of Electrolytes.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Electrodes Of Electrolytes The electrode attached to the negative terminal of a battery is. Electrodes are vital components of electrochemical cells. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Reactive metals are extracted. Electrodes Of Electrolytes.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrodes Of Electrolytes The positive electrode, on the other hand, will attract negative ions (anions). Ionic compounds conduct electricity when molten or in. Electrodes are vital components of electrochemical cells. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Electrode a conductor used to establish electrical contact with a circuit. Electrochemical. Electrodes Of Electrolytes.
From www.youtube.com
6. Electrode and electrolyte defined (HSC chemistry) YouTube Electrodes Of Electrolytes Electrodes are vital components of electrochemical cells. If we place a variable resistance. Ionic compounds conduct electricity when molten or in solution. Electrode a conductor used to establish electrical contact with a circuit. Reactive metals are extracted from their ores using electrolysis. This charge is based off. To start the great journey of electrolytes, we travel back into the history,. Electrodes Of Electrolytes.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Electrodes Of Electrolytes Electrode a conductor used to establish electrical contact with a circuit. Electrodes are vital components of electrochemical cells. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Electrochemical cells allow measurement and control of a redox reaction. If we place a variable resistance. The positive electrode, on the other hand, will attract. Electrodes Of Electrolytes.
From www.snexplores.org
Explainer What is an electrode? Electrodes Of Electrolytes This charge is based off. The positive electrode, on the other hand, will attract negative ions (anions). In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Electrode a conductor used to establish electrical contact with a circuit. To start the great journey of electrolytes, we travel back into the history, and learn. Electrodes Of Electrolytes.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Electrodes Of Electrolytes Electrolyte is the ionic compound in a molten or dissolved solution that conducts. Reactive metals are extracted from their ores using electrolysis. Electrochemical cells allow measurement and control of a redox reaction. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. This charge is based off. Electrode is. Electrodes Of Electrolytes.
From www.researchgate.net
Electrochemical performance of electrodes in different electrolytes. a Electrodes Of Electrolytes This charge is based off. Electrochemical cells allow measurement and control of a redox reaction. Ionic compounds conduct electricity when molten or in solution. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Electrode is a rod of metal or graphite through which an electric current flows into. Electrodes Of Electrolytes.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrodes Of Electrolytes This charge is based off. Electrode a conductor used to establish electrical contact with a circuit. Reactive metals are extracted from their ores using electrolysis. Electrolyte is the ionic compound in a molten or dissolved solution that conducts. Ionic compounds conduct electricity when molten or in solution. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs. Electrodes Of Electrolytes.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science Electrodes Of Electrolytes Reactive metals are extracted from their ores using electrolysis. Electrochemical cells allow measurement and control of a redox reaction. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Ionic compounds conduct electricity when molten or in solution. Electrolyte is the ionic compound in a molten or dissolved solution. Electrodes Of Electrolytes.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Electrodes Of Electrolytes This charge is based off. Reactive metals are extracted from their ores using electrolysis. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Reactive metals are extracted from their ores using electrolysis. Electrodes are vital components of electrochemical cells. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. Electrodes Of Electrolytes.
From stock.adobe.com
Electrolytic cell infographic diagram with components including anode Electrodes Of Electrolytes Electrodes are vital components of electrochemical cells. Reactive metals are extracted from their ores using electrolysis. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. The reaction can. Electrodes Of Electrolytes.
From www.researchgate.net
ElectrodeElectrolyte Interface Download Scientific Diagram Electrodes Of Electrolytes In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. The electrode attached to the negative terminal of a battery is. Reactive metals are extracted from their ores using electrolysis. Electrochemical cells allow. Electrodes Of Electrolytes.
From innovations.stanford.edu
Measuring electrolytes at home for preventive care Stanford Electrodes Of Electrolytes The positive electrode, on the other hand, will attract negative ions (anions). This charge is based off. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Reactive metals are extracted from their ores using electrolysis. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday. Electrodes Of Electrolytes.
From www.batterypowertips.com
What is an electrolyte? Battery Power Tips Electrodes Of Electrolytes Electrodes are vital components of electrochemical cells. Electrolyte is the ionic compound in a molten or dissolved solution that conducts. This charge is based off. Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. The electrode attached to the negative terminal of a battery is. The reaction can be started and. Electrodes Of Electrolytes.
From www.researchgate.net
Schematic illustration of three types of electrolytes. Download Electrodes Of Electrolytes Ionic compounds conduct electricity when molten or in. The electrode attached to the negative terminal of a battery is. Electrode a conductor used to establish electrical contact with a circuit. Ionic compounds conduct electricity when molten or in solution. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and.. Electrodes Of Electrolytes.
From www.researchgate.net
A schematic diagram of the cathodeelectrolyte interface before and Electrodes Of Electrolytes The electrode attached to the negative terminal of a battery is. The reaction can be started and stopped by connecting or disconnecting the two electrodes. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Reactive metals are extracted from their ores using electrolysis. Electrodes are vital components of. Electrodes Of Electrolytes.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Electrodes Of Electrolytes The electrode attached to the negative terminal of a battery is. Reactive metals are extracted from their ores using electrolysis. Electrode a conductor used to establish electrical contact with a circuit. The positive electrode, on the other hand, will attract negative ions (anions). Reactive metals are extracted from their ores using electrolysis. Electrochemical cells allow measurement and control of a. Electrodes Of Electrolytes.
From wisc.pb.unizin.org
Day 41 Electrolysis; Commercial Batteries Chemistry 109, Fall 2020 Electrodes Of Electrolytes Electrodes are vital components of electrochemical cells. Electrochemical cells allow measurement and control of a redox reaction. The electrode attached to the negative terminal of a battery is. Electrode a conductor used to establish electrical contact with a circuit. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. If we place a. Electrodes Of Electrolytes.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Electrodes Of Electrolytes Reactive metals are extracted from their ores using electrolysis. Electrochemical cells allow measurement and control of a redox reaction. Electrolyte is the ionic compound in a molten or dissolved solution that conducts. Ionic compounds conduct electricity when molten or in. Ionic compounds conduct electricity when molten or in solution. To start the great journey of electrolytes, we travel back into. Electrodes Of Electrolytes.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Electrodes Of Electrolytes The positive electrode, on the other hand, will attract negative ions (anions). Electrolyte is the ionic compound in a molten or dissolved solution that conducts. This charge is based off. Electrodes are vital components of electrochemical cells. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Electrode is a rod of metal or graphite through. Electrodes Of Electrolytes.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Electrodes Of Electrolytes Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Reactive metals are extracted from their ores using electrolysis. Electrolyte is the ionic compound in a molten or dissolved solution that conducts. Electrode a conductor used to establish electrical contact with a circuit. The positive electrode, on the other hand,. Electrodes Of Electrolytes.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrodes Of Electrolytes This charge is based off. Reactive metals are extracted from their ores using electrolysis. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive electrode, on the other hand, will attract negative ions (anions). To start the great journey of electrolytes, we travel back into the history, and learn how the. Electrodes Of Electrolytes.
From www.researchgate.net
1. (a) A schematic diagram of electrochemical double layer, (b) the Electrodes Of Electrolytes Reactive metals are extracted from their ores using electrolysis. The electrode attached to the negative terminal of a battery is. Electrode a conductor used to establish electrical contact with a circuit. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. This charge is based off. If we place a. Electrodes Of Electrolytes.
From www.shutterstock.com
Electrode Electrolyte Half Cell Chemistry Lesson เวกเตอร์สต็อก (ปลอด Electrodes Of Electrolytes Electrolyte is the ionic compound in a molten or dissolved solution that conducts. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Electrode a conductor used to establish electrical contact with a circuit. The positive electrode, on the other hand, will attract negative ions (anions). The reaction can. Electrodes Of Electrolytes.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Electrodes Of Electrolytes Reactive metals are extracted from their ores using electrolysis. Electrode a conductor used to establish electrical contact with a circuit. Electrodes are vital components of electrochemical cells. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Ionic compounds conduct electricity when molten or in. If we place a variable resistance. This charge is based off.. Electrodes Of Electrolytes.
From www.researchgate.net
Schematic of the (negative) electrodeelectrolyte interface in EDLCs Electrodes Of Electrolytes To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Electrode a conductor used to establish electrical contact with a circuit. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Ionic compounds conduct electricity when molten or in. Electrodes Of Electrolytes.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrodes Of Electrolytes Ionic compounds conduct electricity when molten or in. The positive electrode, on the other hand, will attract negative ions (anions). The electrode attached to the negative terminal of a battery is. Reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using electrolysis. Electrode is a rod of metal or graphite through which an. Electrodes Of Electrolytes.
From www.researchgate.net
Schematic of the electrodeelectrolyte interface in electrochemical Electrodes Of Electrolytes Electrodes are vital components of electrochemical cells. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Ionic. Electrodes Of Electrolytes.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Electrodes Of Electrolytes Electrochemical cells allow measurement and control of a redox reaction. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. The positive electrode, on the other hand, will attract negative ions (anions). This charge is based off. Electrode is a rod of metal or graphite through which an electric. Electrodes Of Electrolytes.
From giowrvapv.blob.core.windows.net
What Electrodes Are Used In Electrolysis at Patricia Nutt blog Electrodes Of Electrolytes Ionic compounds conduct electricity when molten or in. Electrolyte is the ionic compound in a molten or dissolved solution that conducts. Electrodes are vital components of electrochemical cells. Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. This charge is based off. Electrode is a rod of metal or graphite through. Electrodes Of Electrolytes.
From www.animalia-life.club
Electrolyte Chemistry Electrodes Of Electrolytes Electrodes are vital components of electrochemical cells. Ionic compounds conduct electricity when molten or in solution. Electrode a conductor used to establish electrical contact with a circuit. The electrode attached to the negative terminal of a battery is. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in. To start the great journey. Electrodes Of Electrolytes.
From www.researchgate.net
Classification of electrolytes for electrochemical supercapacitors Electrodes Of Electrolytes If we place a variable resistance. The positive electrode, on the other hand, will attract negative ions (anions). Ionic compounds conduct electricity when molten or in solution. Ionic compounds conduct electricity when molten or in. This charge is based off. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday. Electrodes Of Electrolytes.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrodes Of Electrolytes Electrolyte is the ionic compound in a molten or dissolved solution that conducts. If we place a variable resistance. Electrochemical cells allow measurement and control of a redox reaction. Ionic compounds conduct electricity when molten or in solution. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The positive electrode, on the. Electrodes Of Electrolytes.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps Electrodes Of Electrolytes Electrode a conductor used to establish electrical contact with a circuit. The reaction can be started and stopped by connecting or disconnecting the two electrodes. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Reactive metals are extracted from their ores using electrolysis. Electrochemical cells allow measurement and. Electrodes Of Electrolytes.