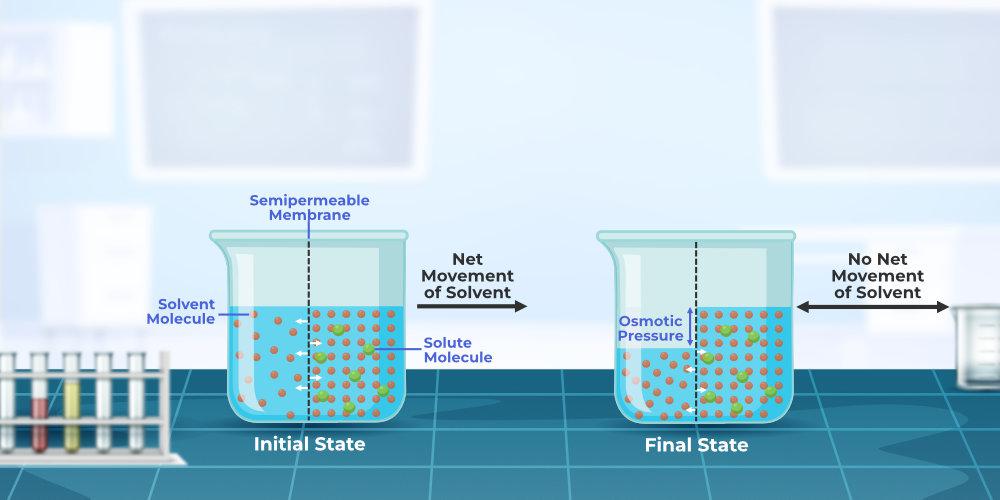
Osmotic Pressure Osmosis . The pressure required to halt the. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. This tendency is called osmotic pressure. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. External pressure can be exerted on a solution to counter the flow of solvent; The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. Osmotic pressure obeys a law that resembles the ideal gas.
from dauglas.afphila.com
The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. External pressure can be exerted on a solution to counter the flow of solvent; The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. This tendency is called osmotic pressure. The pressure required to halt the. Osmotic pressure obeys a law that resembles the ideal gas.
Osmosis Definition, Osmotic Pressure, Formula, Examples, & FAQs
Osmotic Pressure Osmosis This tendency is called osmotic pressure. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. External pressure can be exerted on a solution to counter the flow of solvent; This tendency is called osmotic pressure. Osmotic pressure obeys a law that resembles the ideal gas. The pressure required to halt the. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps Osmotic Pressure Osmosis Osmotic pressure obeys a law that resembles the ideal gas. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. External pressure can be exerted on a solution to counter the flow of solvent; The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. Osmotic pressure. Osmotic Pressure Osmosis.
From www.priyamstudycentre.com
Osmosis Definition, Example, Osmotic Pressure Osmotic Pressure Osmosis This tendency is called osmotic pressure. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. External pressure can be exerted on a solution to counter the flow of solvent; Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. Osmotic pressure obeys a law. Osmotic Pressure Osmosis.
From www.studypool.com
SOLUTION Osmosis and osmotic pressure Studypool Osmotic Pressure Osmosis External pressure can be exerted on a solution to counter the flow of solvent; The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. Osmotic pressure can be defined as the minimum pressure that must. Osmotic Pressure Osmosis.
From www.slideserve.com
PPT Importance of Osmosis and Osmotic Pressure PowerPoint Osmotic Pressure Osmosis The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. The pressure required to halt the. This tendency is called osmotic pressure. Osmotic pressure can be defined as the. Osmotic Pressure Osmosis.
From sciencenotes.org
Osmotic Pressure Definition, Formula, Examples Osmotic Pressure Osmosis External pressure can be exerted on a solution to counter the flow of solvent; The pressure required to halt the. This tendency is called osmotic pressure. The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. Osmotic pressure can be defined as the minimum pressure that must be applied to. Osmotic Pressure Osmosis.
From www.chemistrylearner.com
Osmotic Pressure Definition, Formula, and Examples Osmotic Pressure Osmosis The pressure required to halt the. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. Osmotic pressure obeys a law that resembles the ideal gas. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules. Osmotic Pressure Osmosis.
From shaunmwilliams.com
Chapter 11 Presentation Osmotic Pressure Osmosis The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure obeys a law that resembles the ideal gas. External pressure can be exerted on a solution to counter the flow of solvent; The pressure. Osmotic Pressure Osmosis.
From www.youtube.com
Osmosis Osmotic Pressure YouTube Osmotic Pressure Osmosis This tendency is called osmotic pressure. Osmotic pressure obeys a law that resembles the ideal gas. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. The pressure required to halt the. The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. Osmotic pressure can be. Osmotic Pressure Osmosis.
From www.researchgate.net
Schematic diagram of a osmosis (buildup of osmotic pressure Osmotic Pressure Osmosis This tendency is called osmotic pressure. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. Osmotic pressure obeys a law. Osmotic Pressure Osmosis.
From www.shutterstock.com
Osmotic Pressure Osmosis Reverse Solvent Solute Stock Illustration Osmotic Pressure Osmosis The pressure required to halt the. This tendency is called osmotic pressure. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. Osmotic pressure can be defined as the minimum pressure that must be applied to. Osmotic Pressure Osmosis.
From www.youtube.com
12C02 Solutions Osmosis & Osmotic Pressure Solutions CBSE/IIT Osmotic Pressure Osmosis The pressure required to halt the. This tendency is called osmotic pressure. Osmotic pressure obeys a law that resembles the ideal gas. The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt. Osmotic Pressure Osmosis.
From www.britannica.com
Osmosis Definition, Examples, & Facts Britannica Osmotic Pressure Osmosis Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). The osmotic pressure of a solution depends on the concentration of dissolved solute particles. External pressure can be exerted on a solution to counter the flow of solvent; The pressure required to. Osmotic Pressure Osmosis.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps Osmotic Pressure Osmosis The pressure required to halt the. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). Osmotic pressure obeys a law that resembles the ideal gas. Osmotic pressure. Osmotic Pressure Osmosis.
From www.carlsonstockart.com
Osmosis Carlson Stock Art Osmotic Pressure Osmosis The pressure required to halt the. Osmotic pressure obeys a law that resembles the ideal gas. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. External pressure can be exerted on a solution to. Osmotic Pressure Osmosis.
From www.coastalwiki.org
Osmosis Coastal Wiki Osmotic Pressure Osmosis External pressure can be exerted on a solution to counter the flow of solvent; The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be defined as the minimum pressure that must be. Osmotic Pressure Osmosis.
From www.sciencefacts.net
Osmosis Definition and How Does it Occur (with Diagram) Osmotic Pressure Osmosis Osmotic pressure obeys a law that resembles the ideal gas. The pressure required to halt the. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. This tendency is called osmotic pressure. Osmotic pressure can. Osmotic Pressure Osmosis.
From courses.lumenlearning.com
9.4 Properties of Solutions Osmosis The Basics of General, Organic Osmotic Pressure Osmosis The pressure required to halt the. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. The osmotic pressure of a solution. Osmotic Pressure Osmosis.
From www.youtube.com
Osmosis & Osmotic pressure YouTube Osmotic Pressure Osmosis Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. Osmotic pressure can be thought of as the pressure that would be. Osmotic Pressure Osmosis.
From pharmaceuticalmicrobiologi.blogspot.com
PHARMACEUTICAL MICROBIOLOGY Osmotic Pressure Osmotic Pressure Osmosis Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. This tendency is called osmotic. Osmotic Pressure Osmosis.
From www.shutterstock.com
Demonstration Osmotic Pressure Caused By Osmosis Stock Vector (Royalty Osmotic Pressure Osmosis The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure obeys a law that resembles the ideal gas. External pressure can be exerted on a solution to counter the flow of solvent; This tendency is called osmotic pressure. Osmotic pressure can be thought of as the pressure that would be required to stop water. Osmotic Pressure Osmosis.
From sciencevivid.com
Osmosis Introduction, Types, Solution, Osmotic pressure, Factors Osmotic Pressure Osmosis Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). The pressure required to halt the. External pressure can be exerted. Osmotic Pressure Osmosis.
From www.youtube.com
osmosis and osmotic pressure solutions solutions class 12 chemistry Osmotic Pressure Osmosis Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. This tendency is called osmotic pressure. The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. The pressure required to halt the. Osmotic pressure obeys a law that resembles. Osmotic Pressure Osmosis.
From www.youtube.com
Osmotic Pressure Osmosis Colligative property Physiology Series Osmotic Pressure Osmosis External pressure can be exerted on a solution to counter the flow of solvent; The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). Osmotic pressure obeys a. Osmotic Pressure Osmosis.
From www.slideserve.com
PPT Importance of Osmosis and Osmotic Pressure PowerPoint Osmotic Pressure Osmosis The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. Osmotic pressure obeys a law that resembles the ideal gas. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution. Osmotic Pressure Osmosis.
From www.thoughtco.com
Osmotic Pressure and Tonicity Osmotic Pressure Osmosis The pressure required to halt the. External pressure can be exerted on a solution to counter the flow of solvent; Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). Osmotic pressure obeys a law that resembles the ideal gas. This tendency. Osmotic Pressure Osmosis.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID1433362 Osmotic Pressure Osmosis Osmotic pressure obeys a law that resembles the ideal gas. This tendency is called osmotic pressure. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. The pressure required to halt the. External pressure can be exerted on a solution to counter the flow of solvent; Osmotic pressure can be thought of as the pressure that. Osmotic Pressure Osmosis.
From www.slideserve.com
PPT Osmosis and Osmotic Pressure PowerPoint Presentation, free Osmotic Pressure Osmosis The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. External pressure can be exerted. Osmotic Pressure Osmosis.
From psiberg.com
Osmotic Pressure Determination and Applications Osmotic Pressure Osmosis The pressure required to halt the. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. Osmotic pressure can be thought of. Osmotic Pressure Osmosis.
From quizlet.com
Osmosis + Osmotic Pressure Diagram Quizlet Osmotic Pressure Osmosis This tendency is called osmotic pressure. External pressure can be exerted on a solution to counter the flow of solvent; Osmotic pressure obeys a law that resembles the ideal gas. The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. The pressure required to halt the. Osmotic pressure can be. Osmotic Pressure Osmosis.
From www.buzzle.com
Osmosis Vs. Diffusion How are They Different From Each Other? Osmotic Pressure Osmosis This tendency is called osmotic pressure. The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. External pressure can be exerted on a solution to counter the flow of. Osmotic Pressure Osmosis.
From preparatorychemistry.com
Osmosis Osmotic Pressure Osmosis Osmotic pressure obeys a law that resembles the ideal gas. External pressure can be exerted on a solution to counter the flow of solvent; The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier. This. Osmotic Pressure Osmosis.
From www.chemistryland.com
Colligative Properties Osmotic Pressure Osmosis External pressure can be exerted on a solution to counter the flow of solvent; The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. This tendency is called osmotic pressure. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be thought of. Osmotic Pressure Osmosis.
From www.geeksforgeeks.org
What Is Osmosis? Definition, Types, Osmotic Pressure & Significance Osmotic Pressure Osmosis Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). External pressure can be exerted on a solution to counter the flow of solvent; Osmotic pressure obeys a law that resembles the ideal gas. The osmotic pressure of a solution depends on. Osmotic Pressure Osmosis.
From chem.libretexts.org
13.7 Osmotic Pressure Chemistry LibreTexts Osmotic Pressure Osmosis The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic. Osmotic pressure obeys a law that resembles the ideal gas. This tendency is called osmotic pressure. The osmotic pressure of a solution depends on the concentration of dissolved solute particles. Osmotic pressure can be defined as the minimum pressure that. Osmotic Pressure Osmosis.
From dauglas.afphila.com
Osmosis Definition, Osmotic Pressure, Formula, Examples, & FAQs Osmotic Pressure Osmosis The pressure required to halt the. This tendency is called osmotic pressure. Osmotic pressure obeys a law that resembles the ideal gas. Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). The pressure that prevents additional water flow to the more. Osmotic Pressure Osmosis.