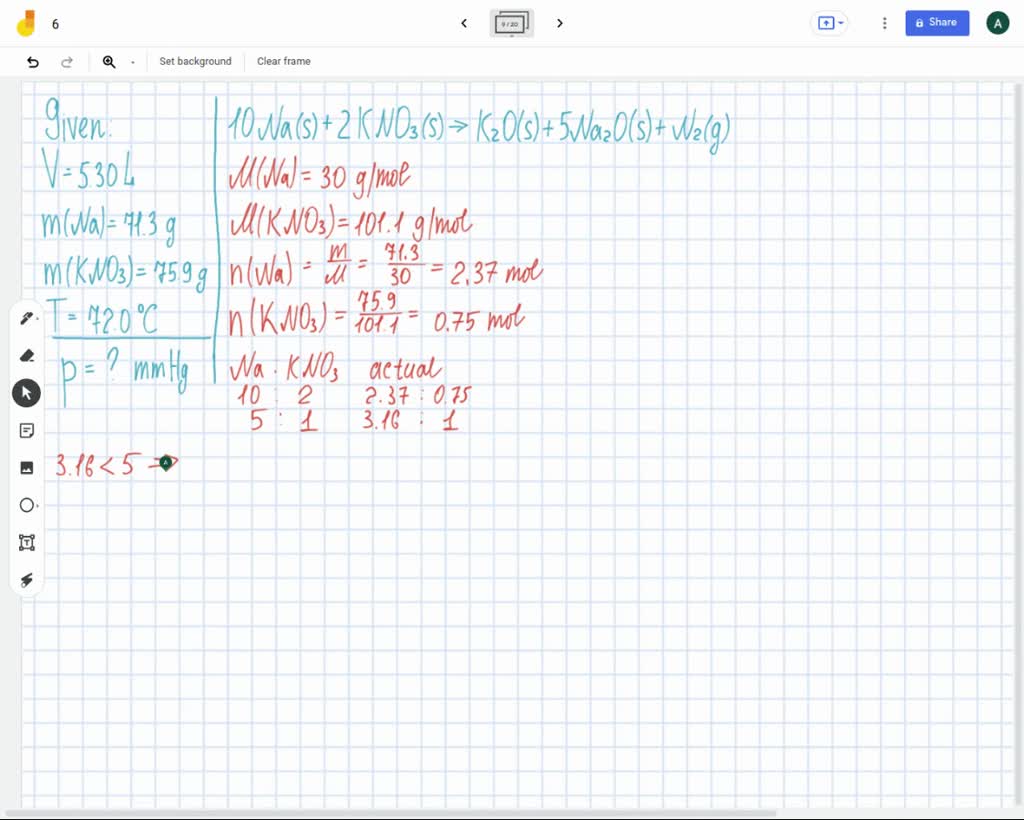
Airbag Chemical Reaction Answer Key . Explore how decomposition reactions are used to inflate an airbag in a car crash. Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on. How does a chemical reaction inflate an airbag? Chemical reactions used to generate the gas. Inside the airbag is a gas generator containing a mixture of nan 3 , kno 3. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Write the reaction for the. If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics should the reagents have? Summary airbags have been shown to. The chemical reaction that occurs in most air bag systems is the decomposition of sodium azide (nan3), producing nitrogen, an extremely unreactive gas. Is this an oxidation reduction reaction or a metathesis reaction? Write and balance the reaction that will occur inside your airbag. Write the balanced chemical equation. Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation.
from www.numerade.com
The chemical reaction that occurs in most air bag systems is the decomposition of sodium azide (nan3), producing nitrogen, an extremely unreactive gas. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Inside the airbag is a gas generator containing a mixture of nan 3 , kno 3. Explore how decomposition reactions are used to inflate an airbag in a car crash. How does a chemical reaction inflate an airbag? Write and balance the reaction that will occur inside your airbag. Write the reaction for the. Is this an oxidation reduction reaction or a metathesis reaction? Summary airbags have been shown to. Chemical reactions used to generate the gas.
SOLVED The inflation of an automobile airbag involves several chemical
Airbag Chemical Reaction Answer Key Explore how decomposition reactions are used to inflate an airbag in a car crash. Write the reaction for the. The chemical reaction that occurs in most air bag systems is the decomposition of sodium azide (nan3), producing nitrogen, an extremely unreactive gas. Explore how decomposition reactions are used to inflate an airbag in a car crash. How does a chemical reaction inflate an airbag? Chemical reactions used to generate the gas. Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation. Inside the airbag is a gas generator containing a mixture of nan 3 , kno 3. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Write the balanced chemical equation. Summary airbags have been shown to. Write and balance the reaction that will occur inside your airbag. If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics should the reagents have? Is this an oxidation reduction reaction or a metathesis reaction? Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on.
From www.scribd.com
POGIL Types of Chemical Reactions PDF Chemical Reactions Chemical Airbag Chemical Reaction Answer Key The chemical reaction that occurs in most air bag systems is the decomposition of sodium azide (nan3), producing nitrogen, an extremely unreactive gas. If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics should the reagents have? Potassium nitrate is used to remove the sodium metal as the second reaction during airbag. Airbag Chemical Reaction Answer Key.
From learningkurugon1.z22.web.core.windows.net
Types Of Reactions Worksheet Key Airbag Chemical Reaction Answer Key If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics should the reagents have? Write the balanced chemical equation. Write and balance the reaction that will occur inside your airbag. Explore how decomposition reactions are used to inflate an airbag in a car crash. How does a chemical reaction inflate an airbag?. Airbag Chemical Reaction Answer Key.
From msoid.ibuypower.com
Types Of Reactions And Balancing Worksheet Best Printable Resources Airbag Chemical Reaction Answer Key Summary airbags have been shown to. If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics should the reagents have? Inside the airbag is a gas generator containing a mixture of nan 3 , kno 3. Write and balance the reaction that will occur inside your airbag. Air bags are activated when. Airbag Chemical Reaction Answer Key.
From www.numerade.com
SOLVED Two further reactions take place in the airbag Reaction A IONa Airbag Chemical Reaction Answer Key How does a chemical reaction inflate an airbag? Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Is this an oxidation reduction reaction or a metathesis reaction? If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics should the. Airbag Chemical Reaction Answer Key.
From www.numerade.com
SOLVED The inflation of an automobile airbag involves several chemical Airbag Chemical Reaction Answer Key Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation. Write and balance the reaction that will occur inside your airbag. Write the balanced chemical equation. The chemical reaction that occurs in most air bag systems is the decomposition of sodium azide (nan3), producing nitrogen, an extremely unreactive gas. How does a chemical reaction. Airbag Chemical Reaction Answer Key.
From www.numerade.com
SOLVEDAirbags can be inflated due to the formation of nitrogen gas in Airbag Chemical Reaction Answer Key Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on. How does a chemical reaction inflate an airbag? The chemical reaction that occurs in most air bag systems is the decomposition of sodium azide (nan3), producing nitrogen, an extremely unreactive gas. Write and balance the reaction that will occur inside your airbag.. Airbag Chemical Reaction Answer Key.
From davida.davivienda.com
Intro To Chemical Reactions Worksheet Answers Printable Word Searches Airbag Chemical Reaction Answer Key Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on. Summary airbags have been shown to. How does a chemical reaction inflate an airbag? Write the balanced chemical equation. Write the reaction for the. Write and balance the reaction that will occur inside your airbag. Chemical reactions used to generate the gas.. Airbag Chemical Reaction Answer Key.
From www.numerade.com
Airbags fill with nitrogen gas from a series of reactions beginning Airbag Chemical Reaction Answer Key Write and balance the reaction that will occur inside your airbag. The chemical reaction that occurs in most air bag systems is the decomposition of sodium azide (nan3), producing nitrogen, an extremely unreactive gas. Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on. Inside the airbag is a gas generator containing. Airbag Chemical Reaction Answer Key.
From studyposter.blogspot.com
Chemistry Study Guide Unit 3 Chemical Reactions Answer Key Study Poster Airbag Chemical Reaction Answer Key Explore how decomposition reactions are used to inflate an airbag in a car crash. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Chemical reactions used to generate the gas. If you wanted to use a different set of chemical reactions to generate gas for airbags, what. Airbag Chemical Reaction Answer Key.
From www.studocu.com
Workbook Unit 5 Chemical Reactions Answer Key CH1120 Studocu Airbag Chemical Reaction Answer Key Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation. Chemical reactions used to generate the gas. Write the balanced chemical equation. Explore how decomposition reactions are used to inflate an airbag in a car crash. If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics. Airbag Chemical Reaction Answer Key.
From www.chegg.com
Solved 3. The reaction that occurs to inflate an airbag in a Airbag Chemical Reaction Answer Key Write the reaction for the. If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics should the reagents have? Write the balanced chemical equation. How does a chemical reaction inflate an airbag? Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on. Write and. Airbag Chemical Reaction Answer Key.
From www.studocu.com
Types of Chemical Reactions Types of Chemical Reactions Types of Airbag Chemical Reaction Answer Key Write and balance the reaction that will occur inside your airbag. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Is this an oxidation reduction reaction or a metathesis reaction? Explore how decomposition reactions are used to inflate an airbag in a car crash. Write the balanced. Airbag Chemical Reaction Answer Key.
From armsingle10.pythonanywhere.com
Glory Airbag Chemical Equation Balanced Equations For Photosynthesis Airbag Chemical Reaction Answer Key Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation. Summary airbags have been shown to. Chemical reactions used to generate the gas. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Write the balanced chemical equation. If you wanted to. Airbag Chemical Reaction Answer Key.
From quizzstaudt.z13.web.core.windows.net
Types Of Chemical Reaction Worksheets Answer Key Airbag Chemical Reaction Answer Key Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation. Write the reaction for. Airbag Chemical Reaction Answer Key.
From www.transtutors.com
(Get Answer) Airbag Lab Data Table 1 Model Air Bag Need Help With Airbag Chemical Reaction Answer Key Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation. How does a chemical reaction inflate an airbag? Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically. Airbag Chemical Reaction Answer Key.
From www.scribd.com
Chemical Reactions Study Guide Answer Key PDF Airbag Chemical Reaction Answer Key Is this an oxidation reduction reaction or a metathesis reaction? Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on. How does a chemical reaction inflate an airbag? Summary airbags have been shown to. Chemical reactions used to generate the gas. Air bags are activated when a severe impact causes a steel. Airbag Chemical Reaction Answer Key.
From answermagicaveri99.z19.web.core.windows.net
Chemical Reaction Worksheet Answer Key Airbag Chemical Reaction Answer Key Chemical reactions used to generate the gas. Write the reaction for the. Write the balanced chemical equation. Write and balance the reaction that will occur inside your airbag. Inside the airbag is a gas generator containing a mixture of nan 3 , kno 3. The chemical reaction that occurs in most air bag systems is the decomposition of sodium azide. Airbag Chemical Reaction Answer Key.
From www.studocu.com
Key Unit 4 Notes Chemical Reactions (2020) What is a Chemical Airbag Chemical Reaction Answer Key Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on. Is this an oxidation reduction reaction or a metathesis reaction? Summary airbags have been shown to. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Write the reaction for. Airbag Chemical Reaction Answer Key.
From www.youtube.com
Airbag and Chemical Reaction Theory YouTube Airbag Chemical Reaction Answer Key If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics should the reagents have? Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation.. Airbag Chemical Reaction Answer Key.
From fyozrjqst.blob.core.windows.net
Lab Simulating A Chemical Reaction Pennies Answer Key at Earl Herr blog Airbag Chemical Reaction Answer Key Is this an oxidation reduction reaction or a metathesis reaction? Write the reaction for the. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Summary airbags have been shown to. Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working. Airbag Chemical Reaction Answer Key.
From www.chegg.com
Solved NEED EXTREME HELP!! When designing an airbag... how Airbag Chemical Reaction Answer Key Chemical reactions used to generate the gas. Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation. Inside the airbag is a gas generator containing a mixture of nan 3 , kno 3. Explore how decomposition reactions are used to inflate an airbag in a car crash. Summary airbags have been shown to. Is. Airbag Chemical Reaction Answer Key.
From www.docsity.com
Worksheet Key for Predicting Products of Chemical Reactions Exercises Airbag Chemical Reaction Answer Key Inside the airbag is a gas generator containing a mixture of nan 3 , kno 3. Chemical reactions used to generate the gas. Write the balanced chemical equation. How does a chemical reaction inflate an airbag? Write and balance the reaction that will occur inside your airbag. Explore how decomposition reactions are used to inflate an airbag in a car. Airbag Chemical Reaction Answer Key.
From learningfullmaurer.z1.web.core.windows.net
Intro To Chemical Reactions Worksheet Answers Airbag Chemical Reaction Answer Key If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics should the reagents have? Summary airbags have been shown to. Inside the airbag is a gas generator containing a mixture of nan 3 , kno 3. How does a chemical reaction inflate an airbag? Explore how decomposition reactions are used to inflate. Airbag Chemical Reaction Answer Key.
From blog.olx.com.pk
How Airbags Work and Save Lives Airbag Chemical Reaction Answer Key Write and balance the reaction that will occur inside your airbag. Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on. If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics should the reagents have? Explore how decomposition reactions are used to inflate an. Airbag Chemical Reaction Answer Key.
From learningcampusbetel.z21.web.core.windows.net
Types Of Chemical Reaction Worksheet Ch 7 Key Airbag Chemical Reaction Answer Key Write the balanced chemical equation. Explore how decomposition reactions are used to inflate an airbag in a car crash. Inside the airbag is a gas generator containing a mixture of nan 3 , kno 3. Write the reaction for the. Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation. Front airbags became mandatory. Airbag Chemical Reaction Answer Key.
From printablefulljanina.z19.web.core.windows.net
Chemical Reactions And Enzymes Worksheet Answers Airbag Chemical Reaction Answer Key Write and balance the reaction that will occur inside your airbag. Summary airbags have been shown to. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics should the reagents. Airbag Chemical Reaction Answer Key.
From www.numerade.com
SOLVED Airbags are present in all new cars being produced today. When Airbag Chemical Reaction Answer Key Write the reaction for the. Chemical reactions used to generate the gas. Summary airbags have been shown to. Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on. How does a chemical reaction inflate an airbag? If you wanted to use a different set of chemical reactions to generate gas for airbags,. Airbag Chemical Reaction Answer Key.
From www.slideserve.com
PPT Balance the following representation of the chemical reaction Airbag Chemical Reaction Answer Key Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation. Write and balance the reaction that will occur inside your airbag. Write the reaction for the. Explore how decomposition reactions are used to inflate an airbag in a car crash. If you wanted to use a different set of chemical reactions to generate gas. Airbag Chemical Reaction Answer Key.
From exoevyktk.blob.core.windows.net
Modeling Chemical Reactions Answer Key at Brittany Hobbs blog Airbag Chemical Reaction Answer Key Is this an oxidation reduction reaction or a metathesis reaction? Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation. Chemical reactions used to generate the gas. Write the reaction for the. The chemical reaction that occurs in most air bag systems is the decomposition of sodium azide (nan3), producing nitrogen, an extremely unreactive. Airbag Chemical Reaction Answer Key.
From www.youtube.com
Airbag The Life saving Chemistry YouTube Airbag Chemical Reaction Answer Key Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Chemical reactions used to generate the gas. Explore how decomposition reactions are used to inflate an airbag in a car crash. Write and balance the reaction that will occur inside your airbag. If you wanted to use a. Airbag Chemical Reaction Answer Key.
From learningconcupy.z21.web.core.windows.net
Types Of Chemical Reaction Worksheet Ch 7 Key Airbag Chemical Reaction Answer Key Write the balanced chemical equation. Is this an oxidation reduction reaction or a metathesis reaction? Inside the airbag is a gas generator containing a mixture of nan 3 , kno 3. If you wanted to use a different set of chemical reactions to generate gas for airbags, what characteristics should the reagents have? Write and balance the reaction that will. Airbag Chemical Reaction Answer Key.
From armsingle10.pythonanywhere.com
Glory Airbag Chemical Equation Balanced Equations For Photosynthesis Airbag Chemical Reaction Answer Key Explore how decomposition reactions are used to inflate an airbag in a car crash. Inside the airbag is a gas generator containing a mixture of nan 3 , kno 3. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Summary airbags have been shown to. Write and. Airbag Chemical Reaction Answer Key.
From materialfullrobin.z13.web.core.windows.net
Introduction To Chemical Reactions Worksheets Answer Key Airbag Chemical Reaction Answer Key Summary airbags have been shown to. Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation. Inside the airbag is a gas generator containing a mixture of nan 3 , kno 3. Explore how decomposition reactions are used to inflate an airbag in a car crash. Write the reaction for the. Is this an. Airbag Chemical Reaction Answer Key.
From www.chegg.com
Solved NEED EXTREME HELP!! When designing an airbag... how Airbag Chemical Reaction Answer Key Is this an oxidation reduction reaction or a metathesis reaction? Front airbags became mandatory on all vehicles in the us in 1999, but car companies started working on. Write the balanced chemical equation. The chemical reaction that occurs in most air bag systems is the decomposition of sodium azide (nan3), producing nitrogen, an extremely unreactive gas. Write and balance the. Airbag Chemical Reaction Answer Key.
From athensmutualaid.net
Chemical Reactions Gizmo Answer Key › Athens Mutual Student Corner Airbag Chemical Reaction Answer Key Potassium nitrate is used to remove the sodium metal as the second reaction during airbag inflation. Summary airbags have been shown to. Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. Write and balance the reaction that will occur inside your airbag. Inside the airbag is a. Airbag Chemical Reaction Answer Key.