Which Of The Following Substances Will Dissolve In Water A Polar Solvent . because water is polar, substances that are polar or ionic will dissolve in it. Learn how water's polarity and hydrogen bonding make it a unique molecule. the electron transfer in ionic compounds results in ions that are attracted to water's poles. Because of the shape of the. water is a versatile solvent that can dissolve many substances. water is called the universal solvent because more substances dissolve in water than in any other chemical. some examples of polar molecules include water and methanal, commonly called. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms.
from www.chegg.com
because water is polar, substances that are polar or ionic will dissolve in it. Learn how water's polarity and hydrogen bonding make it a unique molecule. water is called the universal solvent because more substances dissolve in water than in any other chemical. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. water is a versatile solvent that can dissolve many substances. some examples of polar molecules include water and methanal, commonly called. water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. the electron transfer in ionic compounds results in ions that are attracted to water's poles. Because of the shape of the.
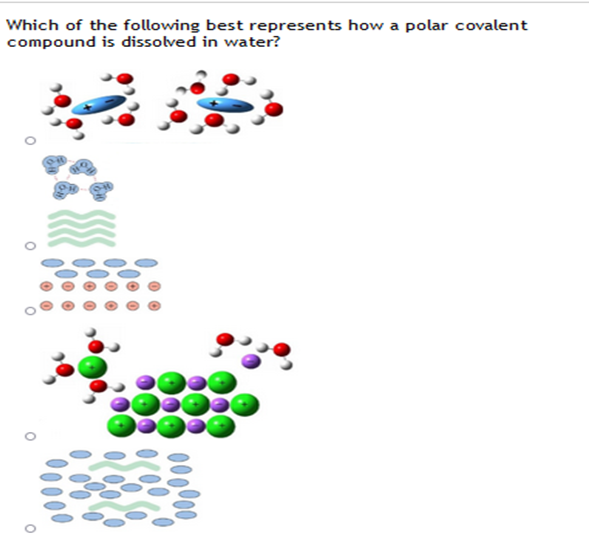
Which of the following best represents how a polar
Which Of The Following Substances Will Dissolve In Water A Polar Solvent water is called the universal solvent because more substances dissolve in water than in any other chemical. because water is polar, substances that are polar or ionic will dissolve in it. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. Learn how water's polarity and hydrogen bonding make it a unique molecule. water is a versatile solvent that can dissolve many substances. some examples of polar molecules include water and methanal, commonly called. water is called the universal solvent because more substances dissolve in water than in any other chemical. Because of the shape of the. the electron transfer in ionic compounds results in ions that are attracted to water's poles. water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms.
From www.numerade.com
SOLVED The following substances dissolve when added to water. Classify Which Of The Following Substances Will Dissolve In Water A Polar Solvent because water is polar, substances that are polar or ionic will dissolve in it. Learn how water's polarity and hydrogen bonding make it a unique molecule. the electron transfer in ionic compounds results in ions that are attracted to water's poles. water is a versatile solvent that can dissolve many substances. some examples of polar molecules. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Which Of The Following Substances Will Dissolve In Water A Polar Solvent Because of the shape of the. water is a versatile solvent that can dissolve many substances. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. some examples of polar molecules include water and methanal, commonly called. water is a polar molecule because of its bent geometry and the electronegativity difference. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From slideplayer.com
Properties of Water. ppt download Which Of The Following Substances Will Dissolve In Water A Polar Solvent some examples of polar molecules include water and methanal, commonly called. water is a versatile solvent that can dissolve many substances. Because of the shape of the. because water is polar, substances that are polar or ionic will dissolve in it. water is called the universal solvent because more substances dissolve in water than in any. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID2493612 Which Of The Following Substances Will Dissolve In Water A Polar Solvent water is a versatile solvent that can dissolve many substances. the electron transfer in ionic compounds results in ions that are attracted to water's poles. Because of the shape of the. because water is polar, substances that are polar or ionic will dissolve in it. water is a polar molecule because of its bent geometry and. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From slideplayer.com
Chapter 2 The Chemistry of Life ppt download Which Of The Following Substances Will Dissolve In Water A Polar Solvent because water is polar, substances that are polar or ionic will dissolve in it. some examples of polar molecules include water and methanal, commonly called. the electron transfer in ionic compounds results in ions that are attracted to water's poles. Learn how water's polarity and hydrogen bonding make it a unique molecule. water is called the. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Which Of The Following Substances Will Dissolve In Water A Polar Solvent Learn how water's polarity and hydrogen bonding make it a unique molecule. the electron transfer in ionic compounds results in ions that are attracted to water's poles. water is a versatile solvent that can dissolve many substances. some examples of polar molecules include water and methanal, commonly called. Because of the shape of the. polar substances. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From nl.pinterest.com
Why Is Water a Polar Molecule? Hydrogen Atom, Hydrogen Bond, Ionic Which Of The Following Substances Will Dissolve In Water A Polar Solvent water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. water is a versatile solvent that can dissolve many substances. water is called the universal solvent because more substances dissolve in water than in any other chemical. Learn how water's polarity and hydrogen bonding make it a. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.numerade.com
SOLVED 'Part C The following substances dissolve when added to water Which Of The Following Substances Will Dissolve In Water A Polar Solvent some examples of polar molecules include water and methanal, commonly called. Because of the shape of the. Learn how water's polarity and hydrogen bonding make it a unique molecule. water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. because water is polar, substances that are polar. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.chegg.com
Which of the following best represents how a polar Which Of The Following Substances Will Dissolve In Water A Polar Solvent Because of the shape of the. the electron transfer in ionic compounds results in ions that are attracted to water's poles. water is called the universal solvent because more substances dissolve in water than in any other chemical. water is a versatile solvent that can dissolve many substances. Learn how water's polarity and hydrogen bonding make it. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From biologydictionary.net
Polar Molecule Definition and Examples Biology Dictionary Which Of The Following Substances Will Dissolve In Water A Polar Solvent because water is polar, substances that are polar or ionic will dissolve in it. the electron transfer in ionic compounds results in ions that are attracted to water's poles. Learn how water's polarity and hydrogen bonding make it a unique molecule. water is a polar molecule because of its bent geometry and the electronegativity difference between the. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID Which Of The Following Substances Will Dissolve In Water A Polar Solvent Because of the shape of the. Learn how water's polarity and hydrogen bonding make it a unique molecule. the electron transfer in ionic compounds results in ions that are attracted to water's poles. because water is polar, substances that are polar or ionic will dissolve in it. some examples of polar molecules include water and methanal, commonly. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Which Of The Following Substances Will Dissolve In Water A Polar Solvent some examples of polar molecules include water and methanal, commonly called. the electron transfer in ionic compounds results in ions that are attracted to water's poles. water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Learn how water's polarity and hydrogen bonding make it a unique. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT Polarity of water PowerPoint Presentation, free download ID2610682 Which Of The Following Substances Will Dissolve In Water A Polar Solvent the electron transfer in ionic compounds results in ions that are attracted to water's poles. because water is polar, substances that are polar or ionic will dissolve in it. water is called the universal solvent because more substances dissolve in water than in any other chemical. some examples of polar molecules include water and methanal, commonly. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From slideplayer.com
Properties of Water Chapter ppt download Which Of The Following Substances Will Dissolve In Water A Polar Solvent water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. water is called the universal solvent because more substances dissolve in water than in any other chemical. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. some examples of polar. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.chegg.com
Solved In which solvent will the following substance Which Of The Following Substances Will Dissolve In Water A Polar Solvent because water is polar, substances that are polar or ionic will dissolve in it. Because of the shape of the. water is a versatile solvent that can dissolve many substances. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. water is called the universal solvent because more substances dissolve in. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From dxonxarcb.blob.core.windows.net
What Type Of Substances Will Dissolve In Water To Form Aqueous Which Of The Following Substances Will Dissolve In Water A Polar Solvent water is a versatile solvent that can dissolve many substances. Learn how water's polarity and hydrogen bonding make it a unique molecule. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. some examples of polar molecules include water and methanal, commonly called. water is a polar molecule because of its. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID269800 Which Of The Following Substances Will Dissolve In Water A Polar Solvent some examples of polar molecules include water and methanal, commonly called. the electron transfer in ionic compounds results in ions that are attracted to water's poles. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. water is a versatile solvent that can dissolve many substances. water is a polar. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.chegg.com
Solved Predict whether each of the following substances Which Of The Following Substances Will Dissolve In Water A Polar Solvent polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. the electron transfer in ionic compounds results in ions that are attracted to water's poles. Learn how water's polarity and hydrogen bonding. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.numerade.com
SOLVED The following substances dissolve when added to water Classify Which Of The Following Substances Will Dissolve In Water A Polar Solvent water is a versatile solvent that can dissolve many substances. some examples of polar molecules include water and methanal, commonly called. the electron transfer in ionic compounds results in ions that are attracted to water's poles. water is called the universal solvent because more substances dissolve in water than in any other chemical. Learn how water's. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From courses.lumenlearning.com
The Dissolution Process Chemistry Which Of The Following Substances Will Dissolve In Water A Polar Solvent Because of the shape of the. the electron transfer in ionic compounds results in ions that are attracted to water's poles. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. Learn how water's polarity and hydrogen bonding make it a unique molecule. because water is polar, substances that are polar or. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT Chapter 2 Water PowerPoint Presentation, free download ID262198 Which Of The Following Substances Will Dissolve In Water A Polar Solvent Learn how water's polarity and hydrogen bonding make it a unique molecule. some examples of polar molecules include water and methanal, commonly called. water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From printableabstenienyg.z22.web.core.windows.net
What Makes Water The Universal Solvent Which Of The Following Substances Will Dissolve In Water A Polar Solvent water is called the universal solvent because more substances dissolve in water than in any other chemical. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. some examples of polar molecules include water and methanal, commonly called. Because of the shape of the. water is a versatile solvent that can. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From slideplayer.com
Chapter 2 Chemistry ppt download Which Of The Following Substances Will Dissolve In Water A Polar Solvent Learn how water's polarity and hydrogen bonding make it a unique molecule. Because of the shape of the. water is a versatile solvent that can dissolve many substances. because water is polar, substances that are polar or ionic will dissolve in it. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents.. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.chemistrysteps.com
Solubility of Organic Compounds Chemistry Steps Which Of The Following Substances Will Dissolve In Water A Polar Solvent Because of the shape of the. water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. some examples of polar molecules include water and methanal, commonly called. water is called the universal solvent because more substances dissolve in water than in any other chemical. polar substances. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.chegg.com
Solved Water is polar solvent. Sucrose is dissolved in Which Of The Following Substances Will Dissolve In Water A Polar Solvent because water is polar, substances that are polar or ionic will dissolve in it. water is called the universal solvent because more substances dissolve in water than in any other chemical. Because of the shape of the. water is a versatile solvent that can dissolve many substances. the electron transfer in ionic compounds results in ions. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.chegg.com
Solved 27. Predict whether each of the following substances Which Of The Following Substances Will Dissolve In Water A Polar Solvent the electron transfer in ionic compounds results in ions that are attracted to water's poles. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. because water is polar, substances that are polar or ionic will dissolve in it. some examples of polar molecules include water and methanal, commonly called. . Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT Polar water molecules interacting with positive and negative ions Which Of The Following Substances Will Dissolve In Water A Polar Solvent some examples of polar molecules include water and methanal, commonly called. water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. the electron transfer in ionic compounds results in ions that are attracted to water's poles. water is a versatile solvent that can dissolve many substances.. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID269800 Which Of The Following Substances Will Dissolve In Water A Polar Solvent water is a versatile solvent that can dissolve many substances. Learn how water's polarity and hydrogen bonding make it a unique molecule. Because of the shape of the. some examples of polar molecules include water and methanal, commonly called. because water is polar, substances that are polar or ionic will dissolve in it. water is a. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.chegg.com
Solved When the following substances dissolve in water, what Which Of The Following Substances Will Dissolve In Water A Polar Solvent because water is polar, substances that are polar or ionic will dissolve in it. water is a versatile solvent that can dissolve many substances. the electron transfer in ionic compounds results in ions that are attracted to water's poles. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. water. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT SOLUTIONS PowerPoint Presentation, free download ID3208141 Which Of The Following Substances Will Dissolve In Water A Polar Solvent some examples of polar molecules include water and methanal, commonly called. water is called the universal solvent because more substances dissolve in water than in any other chemical. Because of the shape of the. Learn how water's polarity and hydrogen bonding make it a unique molecule. because water is polar, substances that are polar or ionic will. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation ID5875746 Which Of The Following Substances Will Dissolve In Water A Polar Solvent water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. water is called the universal solvent because more substances dissolve in water than in any other chemical. some examples of polar molecules include water and methanal, commonly called. Because of the shape of the. the electron. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.chegg.com
Solved Predict whether each of the following substances Which Of The Following Substances Will Dissolve In Water A Polar Solvent some examples of polar molecules include water and methanal, commonly called. the electron transfer in ionic compounds results in ions that are attracted to water's poles. Learn how water's polarity and hydrogen bonding make it a unique molecule. Because of the shape of the. water is a polar molecule because of its bent geometry and the electronegativity. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Which Of The Following Substances Will Dissolve In Water A Polar Solvent Because of the shape of the. polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. the electron transfer in ionic compounds results in ions that are attracted to water's poles. . Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From learningschooldsbbbb56.z4.web.core.windows.net
Explain How Water Is The Universal Solvent Which Of The Following Substances Will Dissolve In Water A Polar Solvent because water is polar, substances that are polar or ionic will dissolve in it. water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. the electron transfer in ionic compounds results in ions that are attracted to water's poles. some examples of polar molecules include water. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Which Of The Following Substances Will Dissolve In Water A Polar Solvent Because of the shape of the. water is a versatile solvent that can dissolve many substances. because water is polar, substances that are polar or ionic will dissolve in it. some examples of polar molecules include water and methanal, commonly called. water is a polar molecule because of its bent geometry and the electronegativity difference between. Which Of The Following Substances Will Dissolve In Water A Polar Solvent.