Two Examples Of Solid-Liquid Solutions . 15 examples of mixtures of solids with liquids. In the first example, we are analyzing a milk sugar solution, and in the second. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Both in everyday life and in the scientific field, there are very frequent mixtures that involve a. It is a solution formed. The solution occurs when ice is introduced into the liquid and cools it while it dissolves. Table 13.2.1 lists some common examples of gaseous, liquid,. A solution with a strong electrolyte produces multiple charged solutes in solution. Physiological saline (solid in liquid). As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. Ice cooling (solid in liquid). The following two examples will show how to distinguish between solid, liquid, and gas solutions. We need to consider the dissociation process and.
from www.youtube.com
Table 13.2.1 lists some common examples of gaseous, liquid,. It is a solution formed. Both in everyday life and in the scientific field, there are very frequent mixtures that involve a. A solution with a strong electrolyte produces multiple charged solutes in solution. Ice cooling (solid in liquid). The solution occurs when ice is introduced into the liquid and cools it while it dissolves. Physiological saline (solid in liquid). 15 examples of mixtures of solids with liquids. We need to consider the dissociation process and. In the first example, we are analyzing a milk sugar solution, and in the second.
States of matter 🚗💧☁️ Solid, Liquid & Gas Learn with examples YouTube
Two Examples Of Solid-Liquid Solutions 15 examples of mixtures of solids with liquids. The solution occurs when ice is introduced into the liquid and cools it while it dissolves. We need to consider the dissociation process and. Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. The following two examples will show how to distinguish between solid, liquid, and gas solutions. In the first example, we are analyzing a milk sugar solution, and in the second. Ice cooling (solid in liquid). Physiological saline (solid in liquid). A solution with a strong electrolyte produces multiple charged solutes in solution. Both in everyday life and in the scientific field, there are very frequent mixtures that involve a. 15 examples of mixtures of solids with liquids. It is a solution formed. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Table 13.2.1 lists some common examples of gaseous, liquid,.
From www.dreamstime.com
Solution Solid in liquid stock vector. Illustration of mixtures Two Examples Of Solid-Liquid Solutions The solution occurs when ice is introduced into the liquid and cools it while it dissolves. Both in everyday life and in the scientific field, there are very frequent mixtures that involve a. 15 examples of mixtures of solids with liquids. Physiological saline (solid in liquid). Two common examples illustrate the contrast between exothermic and endothermic heats of solution of. Two Examples Of Solid-Liquid Solutions.
From animalia-life.club
Types Of Solution Chemistry Two Examples Of Solid-Liquid Solutions The following two examples will show how to distinguish between solid, liquid, and gas solutions. Table 13.2.1 lists some common examples of gaseous, liquid,. It is a solution formed. Ice cooling (solid in liquid). Physiological saline (solid in liquid). In the first example, we are analyzing a milk sugar solution, and in the second. A solution with a strong electrolyte. Two Examples Of Solid-Liquid Solutions.
From ar.inspiredpencil.com
Examples Of Solid Solutions Two Examples Of Solid-Liquid Solutions Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. Table 13.2.1 lists some common examples of gaseous, liquid,. In the first example, we are analyzing a milk sugar solution, and in the second.. Two Examples Of Solid-Liquid Solutions.
From www.expii.com
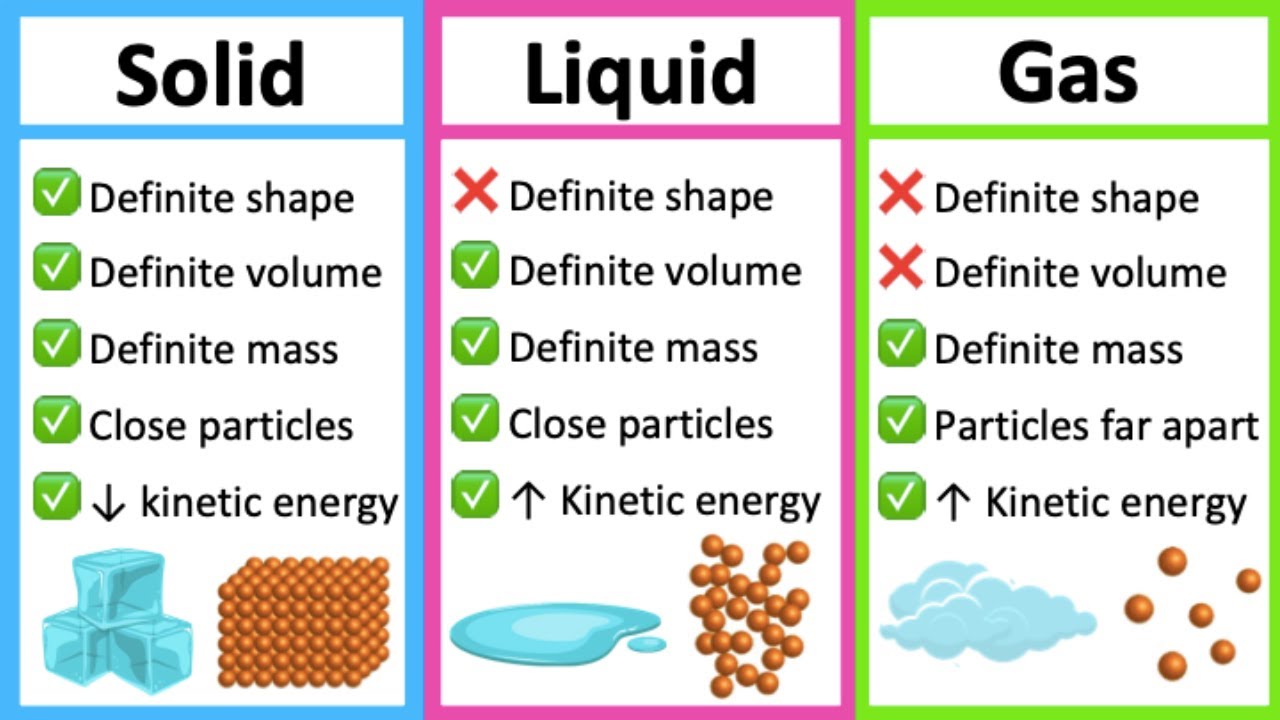
Arrangement of Particles in Phases of Matter — Comparison Expii Two Examples Of Solid-Liquid Solutions In the first example, we are analyzing a milk sugar solution, and in the second. Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. Ice cooling (solid in liquid). We need to consider the dissociation process and. A solution with a strong electrolyte produces multiple charged solutes in solution.. Two Examples Of Solid-Liquid Solutions.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Two Examples Of Solid-Liquid Solutions It is a solution formed. The following two examples will show how to distinguish between solid, liquid, and gas solutions. As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. A solution with a strong electrolyte produces multiple charged solutes in solution. 15 examples of mixtures of. Two Examples Of Solid-Liquid Solutions.
From slideplayer.com
Solutions Chapter ppt download Two Examples Of Solid-Liquid Solutions As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. It is a solution formed. Ice cooling (solid in liquid). 15 examples of mixtures of solids with liquids. The following two examples will show how to distinguish between solid, liquid, and gas solutions. Salt is perhaps the. Two Examples Of Solid-Liquid Solutions.
From www.ase.org.uk
Solids, liquids and gases Two Examples Of Solid-Liquid Solutions Ice cooling (solid in liquid). A solution with a strong electrolyte produces multiple charged solutes in solution. We need to consider the dissociation process and. 15 examples of mixtures of solids with liquids. As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. It is a solution. Two Examples Of Solid-Liquid Solutions.
From www.youtube.com
States of matter 🚗💧☁️ Solid, Liquid & Gas Learn with examples YouTube Two Examples Of Solid-Liquid Solutions The following two examples will show how to distinguish between solid, liquid, and gas solutions. Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. It is a solution formed. 15 examples of mixtures of solids with liquids. In the first example, we are analyzing a milk sugar solution, and. Two Examples Of Solid-Liquid Solutions.
From primaryleap.co.uk
Chemistry Liquids Level 1 activity for kids PrimaryLeap.co.uk Two Examples Of Solid-Liquid Solutions 15 examples of mixtures of solids with liquids. Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. In the first example, we are analyzing a milk sugar solution, and in the second. As long as the solute and solvent combine to give a homogeneous solution, the solute is said. Two Examples Of Solid-Liquid Solutions.
From www.studypool.com
SOLUTION Examples of solid liquid gas Studypool Two Examples Of Solid-Liquid Solutions 15 examples of mixtures of solids with liquids. Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. Physiological saline (solid in liquid). We need to consider the dissociation process and. Table 13.2.1 lists some common examples of gaseous, liquid,. Both in everyday life and in the scientific field, there. Two Examples Of Solid-Liquid Solutions.
From ar.inspiredpencil.com
Examples Of Solid Solutions Two Examples Of Solid-Liquid Solutions Ice cooling (solid in liquid). The following two examples will show how to distinguish between solid, liquid, and gas solutions. We need to consider the dissociation process and. As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. The solution occurs when ice is introduced into the. Two Examples Of Solid-Liquid Solutions.
From fr.dreamstime.com
Illustration Pour Des Changements D'état Entre Solide, Le Liquide Et Le Two Examples Of Solid-Liquid Solutions The solution occurs when ice is introduced into the liquid and cools it while it dissolves. In the first example, we are analyzing a milk sugar solution, and in the second. 15 examples of mixtures of solids with liquids. As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in. Two Examples Of Solid-Liquid Solutions.
From www.dreamstime.com
Solution. Solid In Liquid Stock Vector Image 67386593 Two Examples Of Solid-Liquid Solutions A solution with a strong electrolyte produces multiple charged solutes in solution. As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. Table 13.2.1 lists some common examples of gaseous, liquid,. Both in everyday life and in the scientific field, there are very frequent mixtures that involve. Two Examples Of Solid-Liquid Solutions.
From www.yourdictionary.com
Examples of Homogeneous Mixtures Solid, Liquid and Gas YourDictionary Two Examples Of Solid-Liquid Solutions Physiological saline (solid in liquid). Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. In the first example, we are analyzing a milk sugar solution, and in the second. It is a solution formed. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic. Two Examples Of Solid-Liquid Solutions.
From brunofuga.adv.br
Solution Chemistry Definition, Types, Examples, 52 OFF Two Examples Of Solid-Liquid Solutions Ice cooling (solid in liquid). Both in everyday life and in the scientific field, there are very frequent mixtures that involve a. The following two examples will show how to distinguish between solid, liquid, and gas solutions. The solution occurs when ice is introduced into the liquid and cools it while it dissolves. Table 13.2.1 lists some common examples of. Two Examples Of Solid-Liquid Solutions.
From 139.59.164.119
10 Examples of Solids, Liquids, Gases, and Plasma Two Examples Of Solid-Liquid Solutions Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. Physiological saline (solid in liquid). It is a solution formed. The solution occurs when ice is introduced into the liquid and cools it while it dissolves. As long as the solute and solvent combine to give a homogeneous solution, the. Two Examples Of Solid-Liquid Solutions.
From webmis.highland.cc.il.us
Aqueous Solutions Two Examples Of Solid-Liquid Solutions In the first example, we are analyzing a milk sugar solution, and in the second. The solution occurs when ice is introduced into the liquid and cools it while it dissolves. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: Ice cooling (solid in liquid). A solution with a strong electrolyte produces multiple. Two Examples Of Solid-Liquid Solutions.
From www.youtube.com
States of Matter Solid Liquid Gas States of Matter drawing Different Two Examples Of Solid-Liquid Solutions Table 13.2.1 lists some common examples of gaseous, liquid,. A solution with a strong electrolyte produces multiple charged solutes in solution. We need to consider the dissociation process and. As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. Ice cooling (solid in liquid). The following two. Two Examples Of Solid-Liquid Solutions.
From saylordotorg.github.io
Solids and Liquids Two Examples Of Solid-Liquid Solutions Table 13.2.1 lists some common examples of gaseous, liquid,. It is a solution formed. We need to consider the dissociation process and. Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. The following two examples will show how to distinguish between solid, liquid, and gas solutions. As long as. Two Examples Of Solid-Liquid Solutions.
From primaryleap.co.uk
Chemistry Solutions And Mixtures Level 1 activity for kids Two Examples Of Solid-Liquid Solutions It is a solution formed. Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: A solution with a strong electrolyte produces multiple charged solutes in solution. We need to consider the dissociation process and. Physiological saline (solid in liquid). The solution occurs when ice is introduced into the liquid and cools it while. Two Examples Of Solid-Liquid Solutions.
From slideplayer.com
N39 Properties of Solutions ppt download Two Examples Of Solid-Liquid Solutions 15 examples of mixtures of solids with liquids. As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. Ice cooling (solid in liquid). Physiological saline (solid in. Two Examples Of Solid-Liquid Solutions.
From www.primaryworks.co.uk
PowerPoint KS2 explanation on solids, liquids & gases KS2 primary Two Examples Of Solid-Liquid Solutions As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. The solution occurs when ice is introduced into the liquid and cools it while it dissolves. Physiological saline (solid in liquid). Table 13.2.1 lists some common examples of gaseous, liquid,. 15 examples of mixtures of solids with. Two Examples Of Solid-Liquid Solutions.
From www.solnpharma.com
Difference Between Solid and Liquid Mixing Two Examples Of Solid-Liquid Solutions In the first example, we are analyzing a milk sugar solution, and in the second. The solution occurs when ice is introduced into the liquid and cools it while it dissolves. As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. We need to consider the dissociation. Two Examples Of Solid-Liquid Solutions.
From sciencenotes.org
What Is an Aqueous Solution? Definition and Examples Two Examples Of Solid-Liquid Solutions We need to consider the dissociation process and. The solution occurs when ice is introduced into the liquid and cools it while it dissolves. Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. Physiological saline (solid in liquid). 15 examples of mixtures of solids with liquids. Table 13.2.1 lists. Two Examples Of Solid-Liquid Solutions.
From sciencenotes.org
Liquid Definition Examples of Liquids Two Examples Of Solid-Liquid Solutions As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. The solution occurs when ice is introduced into the liquid and cools it while it dissolves. We need to consider the dissociation process and. Both in everyday life and in the scientific field, there are very frequent. Two Examples Of Solid-Liquid Solutions.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Two Examples Of Solid-Liquid Solutions In the first example, we are analyzing a milk sugar solution, and in the second. Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. We need to consider the dissociation process and. A solution with a strong electrolyte produces multiple charged solutes in solution. Table 13.2.1 lists some common. Two Examples Of Solid-Liquid Solutions.
From www.youtube.com
Solid in liquid solution YouTube Two Examples Of Solid-Liquid Solutions Table 13.2.1 lists some common examples of gaseous, liquid,. As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. 15 examples of mixtures of solids with liquids. We need to consider the dissociation process and. The following two examples will show how to distinguish between solid, liquid,. Two Examples Of Solid-Liquid Solutions.
From www.britannica.com
phase Definition & Facts Britannica Two Examples Of Solid-Liquid Solutions It is a solution formed. The following two examples will show how to distinguish between solid, liquid, and gas solutions. 15 examples of mixtures of solids with liquids. Ice cooling (solid in liquid). Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: In the first example, we are analyzing a milk sugar solution,. Two Examples Of Solid-Liquid Solutions.
From sites.google.com
9/8/15 Miss Day's Science Class Blog Two Examples Of Solid-Liquid Solutions Two common examples illustrate the contrast between exothermic and endothermic heats of solution of ionic solids: We need to consider the dissociation process and. Both in everyday life and in the scientific field, there are very frequent mixtures that involve a. The solution occurs when ice is introduced into the liquid and cools it while it dissolves. The following two. Two Examples Of Solid-Liquid Solutions.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Two Examples Of Solid-Liquid Solutions 15 examples of mixtures of solids with liquids. It is a solution formed. Both in everyday life and in the scientific field, there are very frequent mixtures that involve a. We need to consider the dissociation process and. As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the. Two Examples Of Solid-Liquid Solutions.
From slideplayer.com
Solutions Chapter ppt download Two Examples Of Solid-Liquid Solutions Table 13.2.1 lists some common examples of gaseous, liquid,. It is a solution formed. Ice cooling (solid in liquid). We need to consider the dissociation process and. In the first example, we are analyzing a milk sugar solution, and in the second. 15 examples of mixtures of solids with liquids. Physiological saline (solid in liquid). The solution occurs when ice. Two Examples Of Solid-Liquid Solutions.
From www.youtube.com
SOLUTION OF SOLID IN LIQUID SOLUTIONll Class Two Examples Of Solid-Liquid Solutions The following two examples will show how to distinguish between solid, liquid, and gas solutions. Physiological saline (solid in liquid). Ice cooling (solid in liquid). A solution with a strong electrolyte produces multiple charged solutes in solution. The solution occurs when ice is introduced into the liquid and cools it while it dissolves. We need to consider the dissociation process. Two Examples Of Solid-Liquid Solutions.
From www.snexplores.org
Explainer What are the different states of matter? Two Examples Of Solid-Liquid Solutions Table 13.2.1 lists some common examples of gaseous, liquid,. Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. Both in everyday life and in the scientific field, there are very frequent mixtures that involve a. The solution occurs when ice is introduced into the liquid and cools it while. Two Examples Of Solid-Liquid Solutions.
From devinitionva.blogspot.com
Definition Of Solute Solvent And Solution DEFINITIONVA Two Examples Of Solid-Liquid Solutions The solution occurs when ice is introduced into the liquid and cools it while it dissolves. As long as the solute and solvent combine to give a homogeneous solution, the solute is said to be soluble in the solvent. Both in everyday life and in the scientific field, there are very frequent mixtures that involve a. We need to consider. Two Examples Of Solid-Liquid Solutions.
From materialcampusdecurion.z5.web.core.windows.net
Solids Liquids And Gases Activities Two Examples Of Solid-Liquid Solutions We need to consider the dissociation process and. 15 examples of mixtures of solids with liquids. Salt is perhaps the most essential and widely used flavoring agent and preservative that can be found in the kitchen. Physiological saline (solid in liquid). The solution occurs when ice is introduced into the liquid and cools it while it dissolves. Both in everyday. Two Examples Of Solid-Liquid Solutions.