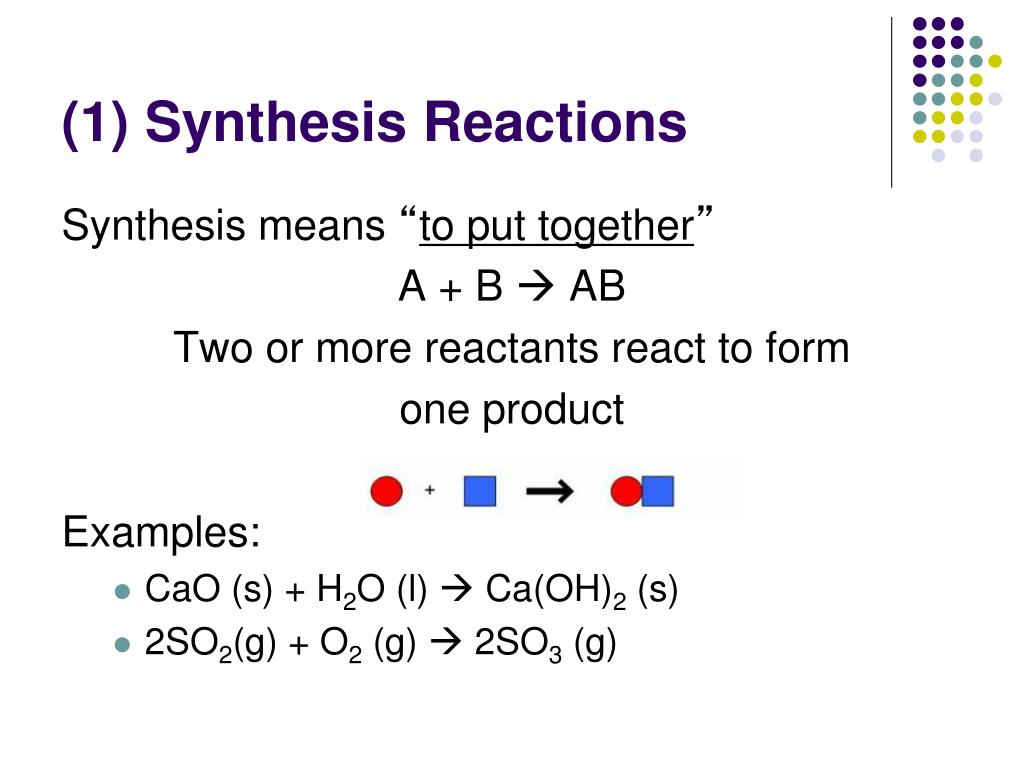
Synthesis Reaction Example In Real Life . In the simplest synthesis reactions, two elements combine to form a binary compound (a. A synthesis reaction is one in which a single product is made from two or more reactants. Production of ammonia (nh3) nitrogen molecules contain two atoms of this element. examples of synthesis reactions. Yes, a common example of a synthesis reaction. can you give an example of a synthesis reaction in real life? there are a few examples of the synthesis reaction in real and daily life. here are some examples of synthesis reactions: 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its.
from www.slideserve.com
2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: A synthesis reaction is one in which a single product is made from two or more reactants. it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. here are some examples of synthesis reactions: there are a few examples of the synthesis reaction in real and daily life. can you give an example of a synthesis reaction in real life? examples of synthesis reactions. In the simplest synthesis reactions, two elements combine to form a binary compound (a. Production of ammonia (nh3) nitrogen molecules contain two atoms of this element. Yes, a common example of a synthesis reaction.
PPT Classifying Chemical Reactions PowerPoint Presentation, free
Synthesis Reaction Example In Real Life Yes, a common example of a synthesis reaction. In the simplest synthesis reactions, two elements combine to form a binary compound (a. Yes, a common example of a synthesis reaction. here are some examples of synthesis reactions: can you give an example of a synthesis reaction in real life? there are a few examples of the synthesis reaction in real and daily life. A synthesis reaction is one in which a single product is made from two or more reactants. Production of ammonia (nh3) nitrogen molecules contain two atoms of this element. examples of synthesis reactions. it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide:
From webapi.bu.edu
🌷 What does synthesis mean in science. What is synthesis chemistry Synthesis Reaction Example In Real Life Production of ammonia (nh3) nitrogen molecules contain two atoms of this element. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. examples of synthesis reactions. there are a few. Synthesis Reaction Example In Real Life.
From www.youtube.com
Synthesis Reactions Reactions) Examples and Practice Synthesis Reaction Example In Real Life examples of synthesis reactions. here are some examples of synthesis reactions: can you give an example of a synthesis reaction in real life? 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: A synthesis reaction is one in which a single product is made from two or more reactants. Production. Synthesis Reaction Example In Real Life.
From www.expii.com
Synthesis Reactions — Definition & Examples Expii Synthesis Reaction Example In Real Life In the simplest synthesis reactions, two elements combine to form a binary compound (a. there are a few examples of the synthesis reaction in real and daily life. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: Yes, a common example of a synthesis reaction. examples of synthesis reactions. here. Synthesis Reaction Example In Real Life.
From www.chemistrylearner.com
Synthesis Reaction Definition, Examples, and Applications Synthesis Reaction Example In Real Life In the simplest synthesis reactions, two elements combine to form a binary compound (a. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. Production of ammonia (nh3) nitrogen molecules contain two. Synthesis Reaction Example In Real Life.
From www.compoundchem.com
Chemical Reactions Posters Part I Compound Interest Synthesis Reaction Example In Real Life can you give an example of a synthesis reaction in real life? it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. In the simplest synthesis reactions, two elements combine to form a binary compound (a. Production of ammonia (nh3) nitrogen molecules contain two atoms of this. Synthesis Reaction Example In Real Life.
From www.slideserve.com
PPT Synthesis Reactions PowerPoint Presentation, free download ID Synthesis Reaction Example In Real Life can you give an example of a synthesis reaction in real life? here are some examples of synthesis reactions: examples of synthesis reactions. A synthesis reaction is one in which a single product is made from two or more reactants. In the simplest synthesis reactions, two elements combine to form a binary compound (a. Production of ammonia. Synthesis Reaction Example In Real Life.
From study.com
Combination Reaction Definition & Examples Video & Lesson Transcript Synthesis Reaction Example In Real Life here are some examples of synthesis reactions: examples of synthesis reactions. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: Yes, a common example of a synthesis reaction. In the simplest synthesis reactions, two elements combine to form a binary compound (a. can you give an example of a synthesis. Synthesis Reaction Example In Real Life.
From www.showme.com
ShowMe synthesis reaction Synthesis Reaction Example In Real Life there are a few examples of the synthesis reaction in real and daily life. can you give an example of a synthesis reaction in real life? A synthesis reaction is one in which a single product is made from two or more reactants. examples of synthesis reactions. Yes, a common example of a synthesis reaction. 2 h. Synthesis Reaction Example In Real Life.
From www.slideserve.com
PPT Synthesis Reaction PowerPoint Presentation, free download ID Synthesis Reaction Example In Real Life Yes, a common example of a synthesis reaction. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: Production of ammonia (nh3) nitrogen molecules contain two atoms of this element. here are some examples of synthesis reactions: there are a few examples of the synthesis reaction in real and daily life. In. Synthesis Reaction Example In Real Life.
From bpdkpomdkf.blogspot.com
Double Replacement Reaction Examples In Real Life All Types Of Synthesis Reaction Example In Real Life examples of synthesis reactions. here are some examples of synthesis reactions: In the simplest synthesis reactions, two elements combine to form a binary compound (a. Yes, a common example of a synthesis reaction. there are a few examples of the synthesis reaction in real and daily life. can you give an example of a synthesis reaction. Synthesis Reaction Example In Real Life.
From sites.google.com
Unit 12 Chemical Reactions Core Physical Science 2019 Synthesis Reaction Example In Real Life A synthesis reaction is one in which a single product is made from two or more reactants. examples of synthesis reactions. there are a few examples of the synthesis reaction in real and daily life. here are some examples of synthesis reactions: In the simplest synthesis reactions, two elements combine to form a binary compound (a. . Synthesis Reaction Example In Real Life.
From www.nagwa.com
Lesson Types of Chemical Reactions Nagwa Synthesis Reaction Example In Real Life 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: Production of ammonia (nh3) nitrogen molecules contain two atoms of this element. A synthesis reaction is one in which a single product is made from two or more reactants. here are some examples of synthesis reactions: examples of synthesis reactions. it. Synthesis Reaction Example In Real Life.
From www.thoughtco.com
Synthesis Reaction Description Plus Examples Synthesis Reaction Example In Real Life examples of synthesis reactions. it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: A synthesis reaction is one in which a single product is made from two or more reactants.. Synthesis Reaction Example In Real Life.
From drcalef.com
Combination (Synthesis) Reactions Synthesis Reaction Example In Real Life here are some examples of synthesis reactions: Yes, a common example of a synthesis reaction. A synthesis reaction is one in which a single product is made from two or more reactants. Production of ammonia (nh3) nitrogen molecules contain two atoms of this element. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon. Synthesis Reaction Example In Real Life.
From www.youtube.com
Predicting products of synthesis reactions YouTube Synthesis Reaction Example In Real Life here are some examples of synthesis reactions: can you give an example of a synthesis reaction in real life? Yes, a common example of a synthesis reaction. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: examples of synthesis reactions. it is easy to find synthesis reaction examples in. Synthesis Reaction Example In Real Life.
From www.vrogue.co
Synthesis Reactions Definition Examples Expii vrogue.co Synthesis Reaction Example In Real Life Production of ammonia (nh3) nitrogen molecules contain two atoms of this element. here are some examples of synthesis reactions: examples of synthesis reactions. there are a few examples of the synthesis reaction in real and daily life. Yes, a common example of a synthesis reaction. 2 h 2 (g) + o 2 (g) → 2 h 2. Synthesis Reaction Example In Real Life.
From sciencenotes.org
What Is a Synthesis Reaction? Definition and Examples Synthesis Reaction Example In Real Life can you give an example of a synthesis reaction in real life? A synthesis reaction is one in which a single product is made from two or more reactants. it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. Production of ammonia (nh3) nitrogen molecules contain two. Synthesis Reaction Example In Real Life.
From www.youtube.com
Types Of Chemical Reactions Synthesis Reactions, Synthesis Reaction Example In Real Life there are a few examples of the synthesis reaction in real and daily life. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: A synthesis reaction is one in which a single product is made from two or more reactants. Yes, a common example of a synthesis reaction. here are some. Synthesis Reaction Example In Real Life.
From www.studocu.com
Synthesis CHEMICAL REACTION A synthesis reaction or direct Synthesis Reaction Example In Real Life examples of synthesis reactions. it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. Yes, a common example of a synthesis reaction. A synthesis reaction is one in which a single product is made from two or more reactants. there are a few examples of the. Synthesis Reaction Example In Real Life.
From www.thoughtco.com
Types of Chemical Reactions (With Examples) Synthesis Reaction Example In Real Life there are a few examples of the synthesis reaction in real and daily life. A synthesis reaction is one in which a single product is made from two or more reactants. here are some examples of synthesis reactions: examples of synthesis reactions. In the simplest synthesis reactions, two elements combine to form a binary compound (a. 2. Synthesis Reaction Example In Real Life.
From trustmyscience.com
Les différents types de réactions chimiques (+exemples) Synthesis Reaction Example In Real Life A synthesis reaction is one in which a single product is made from two or more reactants. can you give an example of a synthesis reaction in real life? there are a few examples of the synthesis reaction in real and daily life. it is easy to find synthesis reaction examples in everyday life, such as the. Synthesis Reaction Example In Real Life.
From www.youtube.com
Reaction Types Synthesis Reaction Example YouTube Synthesis Reaction Example In Real Life Yes, a common example of a synthesis reaction. A synthesis reaction is one in which a single product is made from two or more reactants. there are a few examples of the synthesis reaction in real and daily life. In the simplest synthesis reactions, two elements combine to form a binary compound (a. it is easy to find. Synthesis Reaction Example In Real Life.
From www.pinterest.com
What Is a Reaction? Definition and Examples Life hacks Synthesis Reaction Example In Real Life it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. In the simplest synthesis reactions, two elements combine to form a binary compound (a. here are some examples of synthesis reactions: 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide:. Synthesis Reaction Example In Real Life.
From www.vrogue.co
Synthesis Reactions Definition Examples Expii vrogue.co Synthesis Reaction Example In Real Life it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. examples of synthesis reactions. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: A synthesis reaction is one in which a single product is made from two or more reactants.. Synthesis Reaction Example In Real Life.
From www.slideserve.com
PPT CLASSIFYING CHEMICAL REACTIONS PowerPoint Presentation ID1330610 Synthesis Reaction Example In Real Life Yes, a common example of a synthesis reaction. Production of ammonia (nh3) nitrogen molecules contain two atoms of this element. can you give an example of a synthesis reaction in real life? there are a few examples of the synthesis reaction in real and daily life. A synthesis reaction is one in which a single product is made. Synthesis Reaction Example In Real Life.
From www.haikudeck.com
Synthesis Reaction by Natalie Gayed Synthesis Reaction Example In Real Life examples of synthesis reactions. it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. there are a few examples of the synthesis reaction in real and daily life. Production of ammonia (nh3) nitrogen molecules contain two atoms of this element. A synthesis reaction is one in. Synthesis Reaction Example In Real Life.
From www.thoughtco.com
Examples of Chemical Reactions in Everyday Life Synthesis Reaction Example In Real Life it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. here are some examples of synthesis reactions: examples of synthesis reactions. Yes, a common example of a synthesis reaction. can you give an example of a synthesis reaction in real life? A synthesis reaction is. Synthesis Reaction Example In Real Life.
From www.slideserve.com
PPT Classifying Chemical Reactions PowerPoint Presentation, free Synthesis Reaction Example In Real Life Yes, a common example of a synthesis reaction. examples of synthesis reactions. here are some examples of synthesis reactions: 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. . Synthesis Reaction Example In Real Life.
From www.youtube.com
Amazing Real World Examples of the Basic Chemical Reactions YouTube Synthesis Reaction Example In Real Life Yes, a common example of a synthesis reaction. can you give an example of a synthesis reaction in real life? examples of synthesis reactions. A synthesis reaction is one in which a single product is made from two or more reactants. it is easy to find synthesis reaction examples in everyday life, such as the formation of. Synthesis Reaction Example In Real Life.
From www.thoughtco.com
What Is a Reversible Reaction? Synthesis Reaction Example In Real Life 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: In the simplest synthesis reactions, two elements combine to form a binary compound (a. Yes, a common example of a synthesis reaction. can you give an example of a synthesis reaction in real life? Production of ammonia (nh3) nitrogen molecules contain two atoms. Synthesis Reaction Example In Real Life.
From sciencenotes.org
Types of Chemical Reactions Synthesis Reaction Example In Real Life can you give an example of a synthesis reaction in real life? Production of ammonia (nh3) nitrogen molecules contain two atoms of this element. Yes, a common example of a synthesis reaction. In the simplest synthesis reactions, two elements combine to form a binary compound (a. it is easy to find synthesis reaction examples in everyday life, such. Synthesis Reaction Example In Real Life.
From app.emaze.com
types of chemical reactions on emaze Synthesis Reaction Example In Real Life there are a few examples of the synthesis reaction in real and daily life. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: In the simplest synthesis reactions, two elements combine to form a binary compound (a. can you give an example of a synthesis reaction in real life? it. Synthesis Reaction Example In Real Life.
From www.blendspace.com
Fission And Fusion Lessons Blendspace Synthesis Reaction Example In Real Life here are some examples of synthesis reactions: Yes, a common example of a synthesis reaction. it is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, nacl, from its. can you give an example of a synthesis reaction in real life? 2 h 2 (g) + o 2 (g) →. Synthesis Reaction Example In Real Life.
From www.expii.com
Synthesis Reactions — Definition & Examples Expii Synthesis Reaction Example In Real Life examples of synthesis reactions. A synthesis reaction is one in which a single product is made from two or more reactants. can you give an example of a synthesis reaction in real life? Production of ammonia (nh3) nitrogen molecules contain two atoms of this element. 2 h 2 (g) + o 2 (g) → 2 h 2 o. Synthesis Reaction Example In Real Life.
From www.teachoo.com
Combination Reaction Definition with 4 Examples Class 10 Chemistry Synthesis Reaction Example In Real Life examples of synthesis reactions. can you give an example of a synthesis reaction in real life? 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) carbon dioxide: A synthesis reaction is one in which a single product is made from two or more reactants. it is easy to find synthesis reaction examples. Synthesis Reaction Example In Real Life.