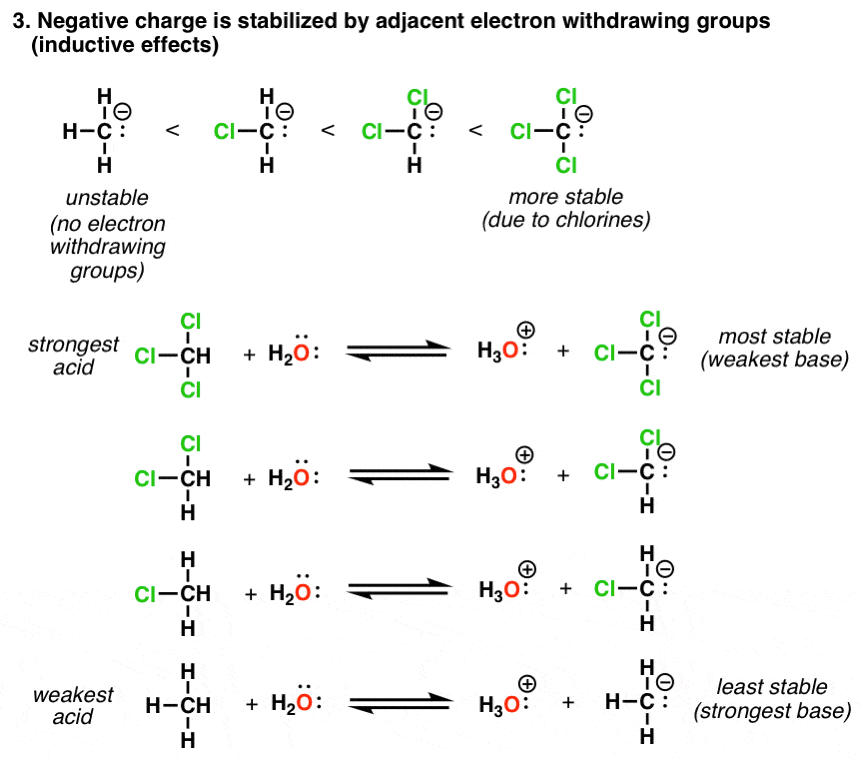
Electron Withdrawing Group Strength . Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This makes the acid more acidic by delocalizing the charge of the. For electron withdrawing groups, all of the sigma complexes are. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal +ve or δ +ve. Ewg = electron withdrawing group; Groups that can donate electron density to the ring make.
from www.masterorganicchemistry.com
Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal +ve or δ +ve. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This makes the acid more acidic by delocalizing the charge of the. For electron withdrawing groups, all of the sigma complexes are. Ewg = electron withdrawing group; Groups that can donate electron density to the ring make.
Acidity Trends In Organic Chemistry Master Organic Chemistry
Electron Withdrawing Group Strength Groups that can donate electron density to the ring make. For electron withdrawing groups, all of the sigma complexes are. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This makes the acid more acidic by delocalizing the charge of the. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal +ve or δ +ve. Ewg = electron withdrawing group; Groups that can donate electron density to the ring make.
From www.reddit.com
[Basic strength] is this universally true or only at ortho and para Electron Withdrawing Group Strength Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Groups that can donate electron density to the ring make. This makes the acid more acidic by delocalizing the charge of the. Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal. Electron Withdrawing Group Strength.
From www.pinterest.com
صورة ذات صلة Organic chemistry, Organic chem, Chemistry Electron Withdrawing Group Strength This makes the acid more acidic by delocalizing the charge of the. Groups that can donate electron density to the ring make. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions.. Electron Withdrawing Group Strength.
From www.researchgate.net
Effect of electron withdrawing or electron donating groups on reduction Electron Withdrawing Group Strength For electron withdrawing groups, all of the sigma complexes are. Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal +ve or δ +ve. This makes the acid more acidic by delocalizing the charge of the. Electron withdrawing groups destabilize the sigma complex and deactivate benzene. Electron Withdrawing Group Strength.
From byjus.com
What is the order (power order)of electron releasing and withdrawing Electron Withdrawing Group Strength This makes the acid more acidic by delocalizing the charge of the. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Ewg can be recognised either by the atom adjacent to. Electron Withdrawing Group Strength.
From achs-prod.acs.org
Role of ElectronDonating and ElectronWithdrawing Groups in Tuning the Electron Withdrawing Group Strength This makes the acid more acidic by delocalizing the charge of the. Groups that can donate electron density to the ring make. Ewg = electron withdrawing group; Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Electron withdrawing groups destabilize the sigma complex and deactivate. Electron Withdrawing Group Strength.
From electronic--racket.blogspot.com
How To Identify Electron Withdrawing And Donating Groups Electron Withdrawing Group Strength Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Ewg = electron withdrawing group; Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal +ve or δ +ve. This makes the. Electron Withdrawing Group Strength.
From www.youtube.com
What is Electron Withdrawing Groups ? Inductive effect & Acidity Electron Withdrawing Group Strength Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having. Electron Withdrawing Group Strength.
From www.masterorganicchemistry.com
EAS On Disubstituted Benzenes The Strongest ElectronDonor "Wins" Electron Withdrawing Group Strength For electron withdrawing groups, all of the sigma complexes are. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This makes the acid more acidic by delocalizing the charge of the. Groups that can donate electron density to the ring make. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites. Electron Withdrawing Group Strength.
From www.clutchprep.com
Electron Withdrawing Groups Organic Chemistry Video Clutch Prep Electron Withdrawing Group Strength This makes the acid more acidic by delocalizing the charge of the. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Groups that can donate electron density to the ring make.. Electron Withdrawing Group Strength.
From www.slideserve.com
PPT Chapter 2 Phenols PowerPoint Presentation, free download ID2962623 Electron Withdrawing Group Strength Ewg = electron withdrawing group; For electron withdrawing groups, all of the sigma complexes are. Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal +ve or δ +ve. Groups that can donate electron density to the ring make. This makes the acid more acidic by. Electron Withdrawing Group Strength.
From www.pinterest.com
ortho meta para summary cheat sheet Chemistry lessons, Organic Electron Withdrawing Group Strength Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Groups that can donate electron density to the ring make. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. For electron withdrawing groups, all of the sigma complexes are. This makes. Electron Withdrawing Group Strength.
From www.pinterest.com
What makes a good leaving group? Organic chemistry, Chemistry Electron Withdrawing Group Strength Ewg = electron withdrawing group; Groups that can donate electron density to the ring make. For electron withdrawing groups, all of the sigma complexes are. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents. Electron Withdrawing Group Strength.
From forums.studentdoctor.net
Resonance Electron Withdrawing or Donating Student Doctor Network Electron Withdrawing Group Strength Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal +ve or δ +ve. This makes the acid more acidic by delocalizing the charge of the. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. For electron withdrawing groups, all of. Electron Withdrawing Group Strength.
From www.researchgate.net
Selected electron‐donating and electron‐withdrawing groups with Electron Withdrawing Group Strength Ewg = electron withdrawing group; Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Groups that can donate electron density to the ring make. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Ewg can be recognised either by the. Electron Withdrawing Group Strength.
From proquestyamaha.web.fc2.com
What is an electron withdrawing group? Electron Withdrawing Group Strength Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. For electron withdrawing groups, all of the sigma complexes are. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This makes the acid more acidic by delocalizing the charge of the.. Electron Withdrawing Group Strength.
From openpress.usask.ca
13.4. Reaction Rates and Relative Reactivity Introduction to Organic Electron Withdrawing Group Strength Groups that can donate electron density to the ring make. For electron withdrawing groups, all of the sigma complexes are. This makes the acid more acidic by delocalizing the charge of the. Ewg = electron withdrawing group; Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to. Electron Withdrawing Group Strength.
From www.chemistrystudent.com
Group 7 Halogens and their Electronegativities (ALevel) ChemistryStudent Electron Withdrawing Group Strength For electron withdrawing groups, all of the sigma complexes are. This makes the acid more acidic by delocalizing the charge of the. Groups that can donate electron density to the ring make. Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal +ve or δ +ve.. Electron Withdrawing Group Strength.
From openpress.usask.ca
10.10. Regioselectivity and Substituent Effects Introduction to Electron Withdrawing Group Strength Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Groups that can donate electron density to the ring make. This makes the acid more acidic by delocalizing the charge of the.. Electron Withdrawing Group Strength.
From www.youtube.com
30.04 Electrondonating and withdrawing Groups YouTube Electron Withdrawing Group Strength Ewg = electron withdrawing group; Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This makes the acid more acidic by delocalizing the charge of the. Groups that can donate electron. Electron Withdrawing Group Strength.
From openpress.usask.ca
13.4. Reaction Rates and Relative Reactivity Introduction to Organic Electron Withdrawing Group Strength Groups that can donate electron density to the ring make. Ewg = electron withdrawing group; Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. For electron withdrawing groups, all of the sigma complexes are. Ewg can be recognised either by the atom adjacent to the. Electron Withdrawing Group Strength.
From forums.studentdoctor.net
Resonance Electron Withdrawing or Donating Student Doctor Network Electron Withdrawing Group Strength Groups that can donate electron density to the ring make. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Ewg = electron withdrawing group; Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. For electron withdrawing groups, all of the. Electron Withdrawing Group Strength.
From www.cadvigyan.com
Electron withdrawing and electron donating capacity CAD Vigyan Electron Withdrawing Group Strength Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Ewg = electron withdrawing group; This makes the acid more acidic by delocalizing the charge of the. For electron withdrawing groups, all. Electron Withdrawing Group Strength.
From www.chemistrysteps.com
Basicity of Amines Chemistry Steps Electron Withdrawing Group Strength Ewg = electron withdrawing group; Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal +ve or δ +ve. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. For electron withdrawing. Electron Withdrawing Group Strength.
From www.toppr.com
Jive reason why electron withdrawing group increases the acidic Electron Withdrawing Group Strength Ewg = electron withdrawing group; For electron withdrawing groups, all of the sigma complexes are. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or,. Electron Withdrawing Group Strength.
From chemguru.sg
2021 P1 Q21 Comparing Basicity of Amines Electron Withdrawing Group Strength This makes the acid more acidic by delocalizing the charge of the. For electron withdrawing groups, all of the sigma complexes are. Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal +ve or δ +ve. Groups that can donate electron density to the ring make.. Electron Withdrawing Group Strength.
From forums.studentdoctor.net
Resonance Electron Withdrawing or Donating Student Doctor Network Electron Withdrawing Group Strength Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Ewg = electron withdrawing group; For electron withdrawing groups, all of the sigma complexes are. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Ewg can be recognised either by the. Electron Withdrawing Group Strength.
From www.masterorganicchemistry.com
Acidity Trends In Organic Chemistry Master Organic Chemistry Electron Withdrawing Group Strength This makes the acid more acidic by delocalizing the charge of the. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Groups that can donate electron density to the ring make. Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal. Electron Withdrawing Group Strength.
From quizlet.com
Electron Withdrawing Groups Diagram Quizlet Electron Withdrawing Group Strength Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This makes the acid more acidic by delocalizing the charge of the. Groups that can donate electron density to the ring make. For electron withdrawing groups, all of the sigma complexes are. Ewg can be recognised either by the atom adjacent to the π system having. Electron Withdrawing Group Strength.
From www.chegg.com
Solved why are ester and amide act as donating group while Electron Withdrawing Group Strength This makes the acid more acidic by delocalizing the charge of the. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Groups that can donate electron density to the ring make. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease.. Electron Withdrawing Group Strength.
From www.pinterest.com
EDGs and EWGs. Organic chemistry, Biochemistry, Electron donating groups Electron Withdrawing Group Strength Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Ewg = electron withdrawing group; This makes the acid more acidic by delocalizing the charge of the. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. Ewg can be recognised either. Electron Withdrawing Group Strength.
From www.masterorganicchemistry.com
Acidity Trends In Organic Chemistry Master Organic Chemistry Electron Withdrawing Group Strength Groups that can donate electron density to the ring make. For electron withdrawing groups, all of the sigma complexes are. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Ewg = electron withdrawing group; This makes the acid more acidic by delocalizing the charge of the. Ewg can be recognised either by the atom adjacent. Electron Withdrawing Group Strength.
From www.clutchprep.com
Electron Withdrawing Groups Organic Chemistry Video Clutch Prep Electron Withdrawing Group Strength Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. For electron withdrawing groups, all of the sigma complexes are. Groups that can donate electron density to the ring make. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Ewg =. Electron Withdrawing Group Strength.
From orgosolver.com
OrgoSolver Electron Withdrawing Group Strength Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal +ve or δ +ve. Ewg = electron withdrawing group; This makes the acid more acidic by delocalizing the charge of the. For electron withdrawing groups, all of the sigma complexes are. Groups that can donate electron. Electron Withdrawing Group Strength.
From byjus.com
Why does electron withdrawing group increases the acidity and releasing Electron Withdrawing Group Strength Ewg can be recognised either by the atom adjacent to the π system having several bonds to more electronegative atoms, or, having a formal +ve or δ +ve. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Groups that can donate electron density to the ring make. This makes the acid more acidic by delocalizing. Electron Withdrawing Group Strength.
From pubs.rsc.org
Influence of the electron donor groups on the optical and Electron Withdrawing Group Strength For electron withdrawing groups, all of the sigma complexes are. Electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Electron withdrawing substituents increase the lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease. This makes the acid more acidic by delocalizing the charge of the.. Electron Withdrawing Group Strength.