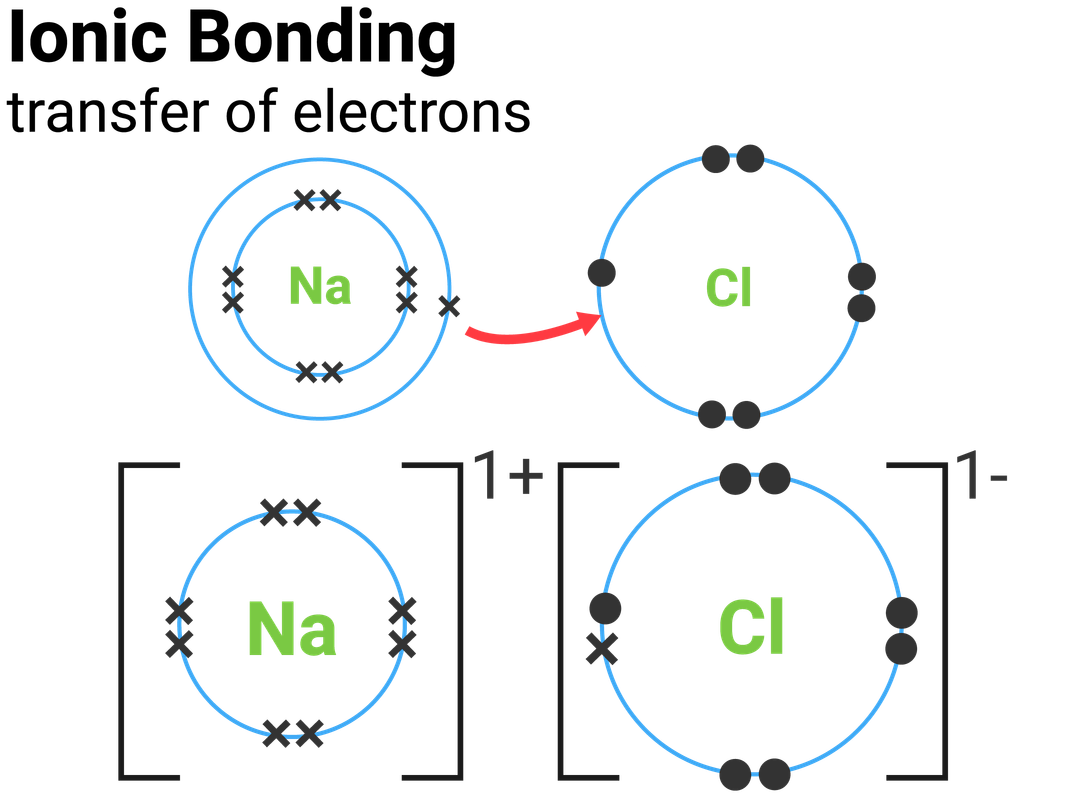
Does Fluorine Form Ionic Bonds . Positive and negative ions form when a. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. These oppositely charged ions attract each other to form ionic networks (or lattices). The polar nature of the. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. Shiny element that is a good conductor of electricity and heat, and which. With other atoms, fluorine forms either polar covalent bonds or ionic bonds.
from revisechemistry.uk
In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. These oppositely charged ions attract each other to form ionic networks (or lattices). Positive and negative ions form when a. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. Shiny element that is a good conductor of electricity and heat, and which. The polar nature of the.
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
Does Fluorine Form Ionic Bonds During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Shiny element that is a good conductor of electricity and heat, and which. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Positive and negative ions form when a. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. The polar nature of the. These oppositely charged ions attract each other to form ionic networks (or lattices). With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Does Fluorine Form Ionic Bonds In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. Shiny element that is a good conductor of electricity and heat, and which. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a.. Does Fluorine Form Ionic Bonds.
From slideplayer.com
ChemistryPart 2 Notes Chemical Bonding ppt download Does Fluorine Form Ionic Bonds During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. These oppositely charged ions attract each other to form ionic networks (or lattices). The polar nature of the. Learn about ionic and covalent bonding,. Does Fluorine Form Ionic Bonds.
From www.slideserve.com
PPT Chemical Bonding PART 1 IONIC PowerPoint Presentation, free Does Fluorine Form Ionic Bonds Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. The high electronegativity of fluorine means that it forms a single electron pair. Does Fluorine Form Ionic Bonds.
From brainybreeze.lighting
What Kind Of Bond Is Formed When Lithium And Fluorine Combine To Form Does Fluorine Form Ionic Bonds The polar nature of the. The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Positive and negative ions form when a. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. These oppositely charged ions attract each other to. Does Fluorine Form Ionic Bonds.
From www.slideserve.com
PPT Stability and Ionic Bonding PowerPoint Presentation ID1443529 Does Fluorine Form Ionic Bonds These oppositely charged ions attract each other to form ionic networks (or lattices). Positive and negative ions form when a. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. In chapter 2 of. Does Fluorine Form Ionic Bonds.
From stock.adobe.com
Ionic compounds formed showing how atoms bond. Sodium and Fluorine Does Fluorine Form Ionic Bonds In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. These oppositely charged ions attract each other to form ionic networks (or lattices). Learn about ionic and covalent bonding, how metals react to form. Does Fluorine Form Ionic Bonds.
From slideplayer.com
CHEMISTRY 161 Chapter 9 Chemical Bonding. ppt download Does Fluorine Form Ionic Bonds The polar nature of the. Shiny element that is a good conductor of electricity and heat, and which. These oppositely charged ions attract each other to form ionic networks (or lattices). In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. With other atoms, fluorine forms either polar covalent bonds or ionic. Does Fluorine Form Ionic Bonds.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Does Fluorine Form Ionic Bonds In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. These oppositely charged ions attract each other to form ionic networks (or lattices). During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. The high electronegativity of fluorine means that it forms a single. Does Fluorine Form Ionic Bonds.
From www.showme.com
Ionic Bonds (Lithium and Fluorine) Chemistry Formulas ShowMe Does Fluorine Form Ionic Bonds With other atoms, fluorine forms either polar covalent bonds or ionic bonds. The polar nature of the. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. Positive and negative ions form when a.. Does Fluorine Form Ionic Bonds.
From learninglab.rmit.edu.au
Covalent bonds Learning Lab Does Fluorine Form Ionic Bonds The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. The polar nature of the. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. Shiny element that is a good conductor of electricity and heat, and which. Learn about ionic and. Does Fluorine Form Ionic Bonds.
From sciencenotes.org
Ionic Bond Definition and Examples Does Fluorine Form Ionic Bonds Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. These oppositely charged ions attract each other to form ionic networks (or lattices). The high electronegativity of fluorine means that it forms a single. Does Fluorine Form Ionic Bonds.
From en.wikipedia.org
Ionic bonding Wikipedia Does Fluorine Form Ionic Bonds Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Shiny element that is a good conductor of electricity and heat, and which. These oppositely charged ions attract each other to form ionic. Does Fluorine Form Ionic Bonds.
From slideplayer.com
Bonding and Chemical Compounds ppt download Does Fluorine Form Ionic Bonds The polar nature of the. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Shiny element that is a good. Does Fluorine Form Ionic Bonds.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Does Fluorine Form Ionic Bonds During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Shiny element that is a good conductor of electricity and heat, and which. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Most frequently, covalent bonds involving fluorine atoms are single bonds,. Does Fluorine Form Ionic Bonds.
From www.youtube.com
Fluorine molecule Animation done with blender 3D covalent bond in Does Fluorine Form Ionic Bonds The polar nature of the. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Positive and negative ions form when a.. Does Fluorine Form Ionic Bonds.
From h-o-m-e.org
The Negative Charge of Fluorine Ions Explained Does Fluorine Form Ionic Bonds During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. The high electronegativity of fluorine. Does Fluorine Form Ionic Bonds.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Does Fluorine Form Ionic Bonds Positive and negative ions form when a. The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Shiny element that is a good conductor of electricity and heat, and which. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties.. Does Fluorine Form Ionic Bonds.
From www.youtube.com
How To Draw The Lewis Structures of Ionic Compounds YouTube Does Fluorine Form Ionic Bonds Positive and negative ions form when a. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. In chapter 2 of this chemistry tutorial, we learned that elements can be classified. Does Fluorine Form Ionic Bonds.
From www.sliderbase.com
Covalent Bonding (Molecules) Presentation Chemistry Does Fluorine Form Ionic Bonds Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. During the formation. Does Fluorine Form Ionic Bonds.
From www.slideserve.com
PPT Bonding PowerPoint Presentation, free download ID6007223 Does Fluorine Form Ionic Bonds During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. These oppositely charged ions attract each other to form ionic networks (or lattices). Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. In chapter 2 of this chemistry tutorial, we learned that. Does Fluorine Form Ionic Bonds.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Does Fluorine Form Ionic Bonds With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. During the formation of some compounds, atoms gain or lose electrons, and. Does Fluorine Form Ionic Bonds.
From www.numerade.com
SOLVED The picture below represents the particle of a compound The Does Fluorine Form Ionic Bonds Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. During the formation of some compounds, atoms gain or lose. Does Fluorine Form Ionic Bonds.
From valenceelectrons.com
Fluorine Electron Configuration With Full Orbital Diagram Does Fluorine Form Ionic Bonds With other atoms, fluorine forms either polar covalent bonds or ionic bonds. The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. The polar nature of the. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. In chapter 2. Does Fluorine Form Ionic Bonds.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Does Fluorine Form Ionic Bonds These oppositely charged ions attract each other to form ionic networks (or lattices). During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Most frequently, covalent bonds involving fluorine atoms are single bonds,. Does Fluorine Form Ionic Bonds.
From quizdbvolcanises.z4.web.core.windows.net
Does Fluorine Form Positive Or Negative Ions Does Fluorine Form Ionic Bonds With other atoms, fluorine forms either polar covalent bonds or ionic bonds. These oppositely charged ions attract each other to form ionic networks (or lattices). The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two. Does Fluorine Form Ionic Bonds.
From www.animalia-life.club
Fluorine Diagram Does Fluorine Form Ionic Bonds During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. The polar nature of the.. Does Fluorine Form Ionic Bonds.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Does Fluorine Form Ionic Bonds In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. The polar nature of the. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. During the formation of some compounds, atoms gain. Does Fluorine Form Ionic Bonds.
From diagramdataselassie.z1.web.core.windows.net
Ionic And Covalent Bonds Venn Diagram Does Fluorine Form Ionic Bonds In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. These oppositely charged ions attract each other to form ionic networks (or lattices). The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. With other atoms, fluorine forms either polar covalent. Does Fluorine Form Ionic Bonds.
From chemnotcheem.com
Notes O Level Chemistry Chem Not Cheem Does Fluorine Form Ionic Bonds Positive and negative ions form when a. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. These oppositely charged ions attract each other to form ionic networks (or lattices). The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. In chapter 2 of this chemistry tutorial,. Does Fluorine Form Ionic Bonds.
From www2.victoriacollege.edu
Chapter 2 Atoms, Molecules and Life Chemistry) Does Fluorine Form Ionic Bonds Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. Shiny element that is a good conductor of electricity and heat, and which. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a. Does Fluorine Form Ionic Bonds.
From schoolbag.info
Figure 3.4. Formation of a Covalent Bond Fluorine (F) has seven valence Does Fluorine Form Ionic Bonds In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. The high electronegativity of fluorine means that it forms a single electron. Does Fluorine Form Ionic Bonds.
From slideplayer.com
Chapter 8 Concepts of Chemical Bonding ppt download Does Fluorine Form Ionic Bonds Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. These oppositely charged ions attract. Does Fluorine Form Ionic Bonds.
From spmchemistry.blog.onlinetuition.com.my
Chemical Bond SPM Chemistry Does Fluorine Form Ionic Bonds During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. Positive and negative ions form when a. These oppositely charged ions attract. Does Fluorine Form Ionic Bonds.
From www.slideserve.com
PPT IONIC BONDING PowerPoint Presentation, free download ID1785078 Does Fluorine Form Ionic Bonds The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. In chapter 2 of this chemistry tutorial, we learned that elements can be classified as metals, nonmetals, and. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. During the. Does Fluorine Form Ionic Bonds.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Does Fluorine Form Ionic Bonds Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a. Shiny element that is a good conductor of electricity and heat, and which. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. The polar nature of the. Positive and negative ions form when a.. Does Fluorine Form Ionic Bonds.