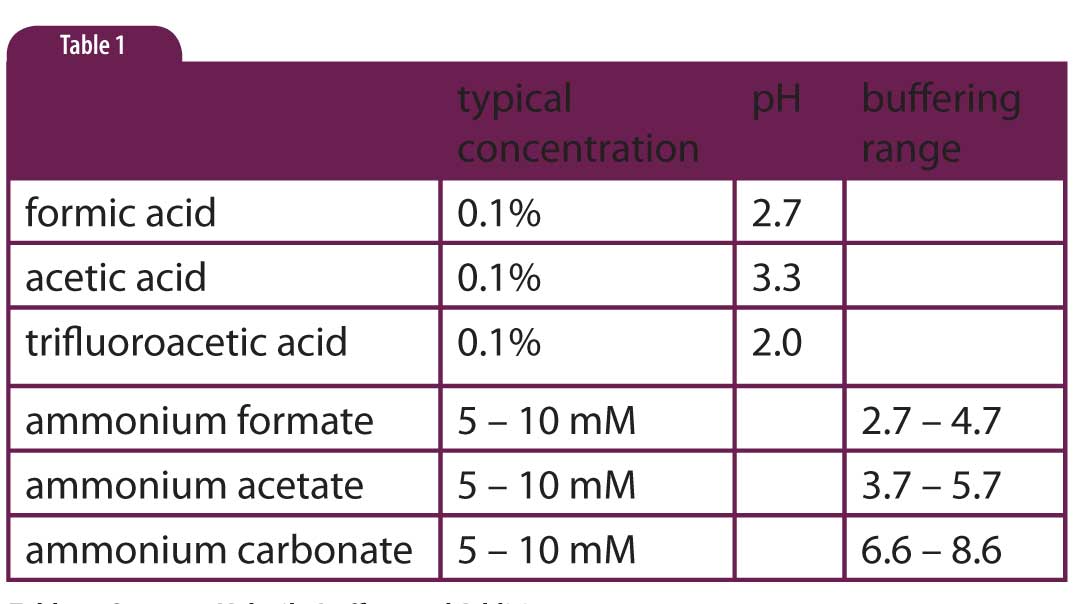
Buffer With Ammonium . a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid. alkaline buffer solutions are commonly made from a weak base and one of its salts. the buffer in this example is made up of nh 4 cl and nh 3. A frequently used example is a mixture of ammonia solution and. Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial.
from blog.sepscience.com
the buffer in this example is made up of nh 4 cl and nh 3. a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid. A frequently used example is a mixture of ammonia solution and. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. alkaline buffer solutions are commonly made from a weak base and one of its salts. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the.
LCMS Be Prepared
Buffer With Ammonium alkaline buffer solutions are commonly made from a weak base and one of its salts. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. alkaline buffer solutions are commonly made from a weak base and one of its salts. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. A frequently used example is a mixture of ammonia solution and. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. the buffer in this example is made up of nh 4 cl and nh 3.
From www.numerade.com
SOLVED A buffer contains significant amounts of ammonia and ammonium Buffer With Ammonium A frequently used example is a mixture of ammonia solution and. a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. another example of a buffer is a solution containing ammonia. Buffer With Ammonium.
From www.researchgate.net
Ammonium chloride phase diagram. Figure reprinted with permission from Buffer With Ammonium Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid. the buffer in this example is made up of nh 4 cl and nh 3. A frequently used example is a mixture of. Buffer With Ammonium.
From www.numerade.com
SOLVEDA buffer contains significant amounts of ammonia and ammonium Buffer With Ammonium the buffer in this example is made up of nh 4 cl and nh 3. Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid. a mixture of ammonia and ammonium chloride. Buffer With Ammonium.
From www.crawfordscientific.com
Ammonium acetate buffers Buffer With Ammonium another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. a buffer is a solution that maintains the stability of a system’s ph. Buffer With Ammonium.
From www.numerade.com
SOLVED Basic buffer solution with pH 10.5 is prepared by dissolving Buffer With Ammonium A frequently used example is a mixture of ammonia solution and. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. alkaline buffer solutions are commonly made from a weak base and one of its salts. a buffer is a solution that maintains the stability of a system’s ph level when adding small. Buffer With Ammonium.
From dramarnathgiri.blogspot.com
EHSQ (Environment,Health,Safety and Quality) Chemistry of buffers and Buffer With Ammonium a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). A frequently used example is a mixture of ammonia solution and. alkaline buffer. Buffer With Ammonium.
From www.coleparmer.com
Reagents Ammonium Chloride/Hydroxide Buffer, 4L, Volumetric from Cole Buffer With Ammonium alkaline buffer solutions are commonly made from a weak base and one of its salts. Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia. Buffer With Ammonium.
From solvedlib.com
(10 points) The ammonium/ 'ammonia buffer system… SolvedLib Buffer With Ammonium Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. another example of a buffer is a. Buffer With Ammonium.
From slideplayer.pl
Hydrolysis & buffers. ppt pobierz Buffer With Ammonium a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the.. Buffer With Ammonium.
From www.fishersci.com
Aqua Solutions Ammonium Acetate Buffer Solution pH 4.6 (1L), Quantity Buffer With Ammonium alkaline buffer solutions are commonly made from a weak base and one of its salts. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. the buffer in this example is made up of nh 4 cl and nh 3. a mixture of. Buffer With Ammonium.
From www.crawfordscientific.com
Ammonium acetate buffers Buffer With Ammonium Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. alkaline buffer solutions are commonly made from a weak base and one of its salts. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. another example of a buffer is a solution containing ammonia (nh 3, a weak base). Buffer With Ammonium.
From www.bio-world.com
Ammonium Acetate Buffer 1M, Sterile bioWORLD Buffer With Ammonium This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl).. Buffer With Ammonium.
From www.sigmaaldrich.com
Ammonium buffer solution for complexometry (ammonium chloride/ammonia Buffer With Ammonium a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. . Buffer With Ammonium.
From www.pinterest.com
Understanding Buffer Solution Chemistry A Complete Guide Buffer With Ammonium Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. the buffer in this example is made up. Buffer With Ammonium.
From sielc.com
Buffer Preparation SIELC Technologies Buffer With Ammonium A frequently used example is a mixture of ammonia solution and. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. alkaline buffer solutions are commonly made from a weak base and one of its salts. the buffer in this example is made up of. Buffer With Ammonium.
From www.electro-glo.com
Hardness Buffer Solution (Ammonia/Ammonium Chloride) ElectroGlo Buffer With Ammonium a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. the buffer in this example is made up of nh 4 cl and nh 3. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. Buffer With Ammonium.
From www.researchgate.net
Effects of ammonium acetate and buffer pH on the migration of analytes Buffer With Ammonium a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. the buffer in this example is made up of nh 4 cl and nh 3. A frequently used example is a mixture of ammonia solution and. alkaline buffer solutions are commonly made from a. Buffer With Ammonium.
From www.researchgate.net
Buffering capacity β of (a) water, (b) ammonia or ammonium, and (c Buffer With Ammonium a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A frequently used example is a mixture of ammonia solution and. alkaline buffer solutions are commonly made from a weak base and one of its salts. Therefore, the acid in the buffer is the ammonium ion. Buffer With Ammonium.
From blog.sepscience.com
LCMS Be Prepared Buffer With Ammonium a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). alkaline buffer solutions are commonly made from a weak base and one of its. Buffer With Ammonium.
From solvedlib.com
(10 points) The ammonium/ 'ammonia buffer system… SolvedLib Buffer With Ammonium Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. . Buffer With Ammonium.
From www.indiamart.com
99 Liquid Ammonia Buffer Solution, Packaging Type Bottle, Packaging Buffer With Ammonium alkaline buffer solutions are commonly made from a weak base and one of its salts. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution. Buffer With Ammonium.
From askfilo.com
An ammonia ammonium chloride buffer has a pH value of 9 with [NH3 ]=0.25.. Buffer With Ammonium another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. alkaline buffer solutions are commonly made from a weak. Buffer With Ammonium.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Buffer With Ammonium another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid. the buffer in this example is made up of nh 4 cl and nh. Buffer With Ammonium.
From www.youtube.com
Buffer solution (Ammonium acetate) YouTube Buffer With Ammonium alkaline buffer solutions are commonly made from a weak base and one of its salts. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. Buffer With Ammonium.
From www.numerade.com
SOLVED A buffer contains significant amounts of ammonia and ammonium Buffer With Ammonium a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a mixture of ammonia and ammonium chloride is basic because the k. Buffer With Ammonium.
From sciencelab.co.ke
Ammonium Buffer PH10 Sciencelab limited Buffer With Ammonium a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. the buffer in this example is made up of nh 4 cl. Buffer With Ammonium.
From courses.lumenlearning.com
Buffers Chemistry Atoms First Buffer With Ammonium a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. the buffer in this example is made up of nh 4 cl and nh 3. Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. This characteristic makes buffers important in biological and. Buffer With Ammonium.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Buffer With Ammonium the buffer in this example is made up of nh 4 cl and nh 3. a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids. Buffer With Ammonium.
From www.chegg.com
Solved 1 A buffer solution contains 0.358 M ammonium Buffer With Ammonium the buffer in this example is made up of nh 4 cl and nh 3. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). A frequently used example is a mixture. Buffer With Ammonium.
From www.reagents.com
Ammonium Acetate Buffer, pH 5 Reagents Buffer With Ammonium a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid. A frequently used example is a mixture of ammonia solution and. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that maintains the stability of. Buffer With Ammonium.
From neutronco.com
Ammonium buffer solution for complementary pH 1110 Neutronco Buffer With Ammonium another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. alkaline buffer solutions are commonly made from a weak base and one of its salts. This characteristic makes buffers important in biological and chemical. Buffer With Ammonium.
From www.numerade.com
SOLVED A buffer contains significant amounts of ammonia and ammonium Buffer With Ammonium This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. alkaline buffer solutions are commonly made from a weak base and one of its salts. A frequently used example is a mixture of ammonia solution and. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater. Buffer With Ammonium.
From www.slideshare.net
02 hydrolysis. buffers__colloids Buffer With Ammonium alkaline buffer solutions are commonly made from a weak base and one of its salts. the buffer in this example is made up of nh 4 cl and nh 3. a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid. a mixture of ammonia. Buffer With Ammonium.
From www.researchgate.net
Effect of (A) acetonitrile content, (B) pH of ammonium formate buffer Buffer With Ammonium Therefore, the acid in the buffer is the ammonium ion (nh 4 +),. a buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid. a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the.. Buffer With Ammonium.
From www.calpaclab.com
Acetic AcidAmmonium Acetate Buffer TS, 500mL Buffer With Ammonium a mixture of ammonia and ammonium chloride is basic because the k b for ammonia is greater than the k a for the. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a mixture of ammonia and ammonium chloride is basic because the k. Buffer With Ammonium.