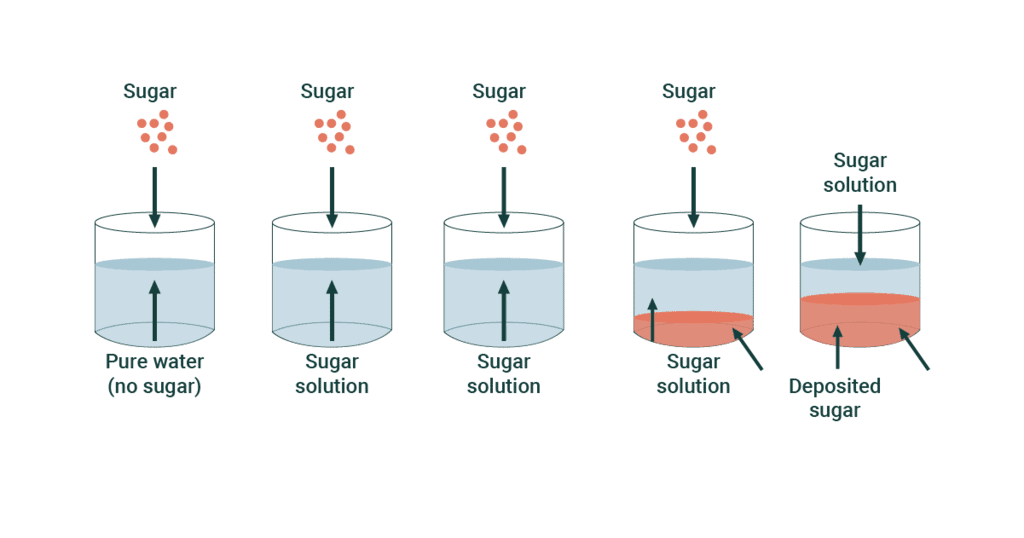
Does Increasing Pressure Increase Solubility . Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also. The solubility of gases is significantly affected by pressure, as described by henry’s law. An increase in pressure and an. At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? This fundamental principle is crucial in understanding various phenomena, from the fizzing. Increasing pressure increased solubility, but increasing temperature decreases solubility; State henry’s law and use it in calculations involving the solubility of a gas in. Describe the effects of temperature and pressure on solubility. The definition of solubility is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature or pressure (in the case of. Many compounds (such as glucose and ch 3 co 2 na) exhibit a dramatic increase in solubility with increasing temperature. Others (such as nacl and k 2 so 4) exhibit little variation, and. The solubility is a measure of the concentration of the dissolved gas particles in the liquid and is a function of the gas pressure. As you increase the pressure of a gas, the collision frequency.
from edurev.in
Describe the effects of temperature and pressure on solubility. An increase in pressure and an. As you increase the pressure of a gas, the collision frequency. The solubility of gases is significantly affected by pressure, as described by henry’s law. At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? State henry’s law and use it in calculations involving the solubility of a gas in. The solubility is a measure of the concentration of the dissolved gas particles in the liquid and is a function of the gas pressure. The definition of solubility is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature or pressure (in the case of. This fundamental principle is crucial in understanding various phenomena, from the fizzing. Increasing pressure increased solubility, but increasing temperature decreases solubility;
Solubility Solutions Class 12 Notes EduRev
Does Increasing Pressure Increase Solubility At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? As you increase the pressure of a gas, the collision frequency. Others (such as nacl and k 2 so 4) exhibit little variation, and. State henry’s law and use it in calculations involving the solubility of a gas in. At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also. The solubility is a measure of the concentration of the dissolved gas particles in the liquid and is a function of the gas pressure. Describe the effects of temperature and pressure on solubility. The definition of solubility is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature or pressure (in the case of. Increasing pressure increased solubility, but increasing temperature decreases solubility; This fundamental principle is crucial in understanding various phenomena, from the fizzing. An increase in pressure and an. Many compounds (such as glucose and ch 3 co 2 na) exhibit a dramatic increase in solubility with increasing temperature. The solubility of gases is significantly affected by pressure, as described by henry’s law.
From www.vedantu.com
What is the relationship between the solubility and the temperature Does Increasing Pressure Increase Solubility Many compounds (such as glucose and ch 3 co 2 na) exhibit a dramatic increase in solubility with increasing temperature. Increasing pressure increased solubility, but increasing temperature decreases solubility; State henry’s law and use it in calculations involving the solubility of a gas in. As you increase the pressure of a gas, the collision frequency. The solubility is a measure. Does Increasing Pressure Increase Solubility.
From chem.libretexts.org
13.4 Pressure and Temperature Effects on Solubility Chemistry LibreTexts Does Increasing Pressure Increase Solubility At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also. State henry’s law and use it in calculations involving the solubility of a. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Chapter 13 Mixtures at the Molecular Level Properties of Does Increasing Pressure Increase Solubility As you increase the pressure of a gas, the collision frequency. At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? The solubility of gases is significantly affected by pressure, as described by henry’s law. Many compounds (such as glucose and ch 3 co 2 na) exhibit a dramatic. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID2516429 Does Increasing Pressure Increase Solubility The solubility of gases is significantly affected by pressure, as described by henry’s law. Others (such as nacl and k 2 so 4) exhibit little variation, and. As you increase the pressure of a gas, the collision frequency. Describe the effects of temperature and pressure on solubility. At constant pressure, what generally happens to the solubility of solids and gases. Does Increasing Pressure Increase Solubility.
From www.reddit.com
[Chemistry] How does intermolecular forces relate to how the solubility Does Increasing Pressure Increase Solubility An increase in pressure and an. This fundamental principle is crucial in understanding various phenomena, from the fizzing. Increasing pressure increased solubility, but increasing temperature decreases solubility; The solubility of gases is significantly affected by pressure, as described by henry’s law. At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Factors Affecting Solubility PowerPoint Presentation, free Does Increasing Pressure Increase Solubility State henry’s law and use it in calculations involving the solubility of a gas in. Others (such as nacl and k 2 so 4) exhibit little variation, and. This fundamental principle is crucial in understanding various phenomena, from the fizzing. Increasing pressure increased solubility, but increasing temperature decreases solubility; An increase in pressure and an. Because the concentration of molecules. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Solubility Notes PowerPoint Presentation, free download ID5606676 Does Increasing Pressure Increase Solubility Describe the effects of temperature and pressure on solubility. Increasing pressure increased solubility, but increasing temperature decreases solubility; Others (such as nacl and k 2 so 4) exhibit little variation, and. Many compounds (such as glucose and ch 3 co 2 na) exhibit a dramatic increase in solubility with increasing temperature. At constant pressure, what generally happens to the solubility. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 Does Increasing Pressure Increase Solubility The solubility of gases is significantly affected by pressure, as described by henry’s law. At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID3105812 Does Increasing Pressure Increase Solubility At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? The solubility of gases is significantly affected by pressure, as described by henry’s law. Others (such as nacl and k 2 so 4) exhibit little variation, and. An increase in pressure and an. The solubility is a measure of. Does Increasing Pressure Increase Solubility.
From www.slideshare.net
Factors Affecting Solubility Does Increasing Pressure Increase Solubility Describe the effects of temperature and pressure on solubility. The definition of solubility is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature or pressure (in the case of. The solubility of gases is significantly affected by pressure, as described by henry’s law. At constant pressure, what. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2012082 Does Increasing Pressure Increase Solubility Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also. At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? As you increase the pressure of a gas, the collision frequency. Many compounds. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT AQUEOUS SOLUTIONS PowerPoint Presentation, free download ID1197096 Does Increasing Pressure Increase Solubility Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also. An increase in pressure and an. The solubility is a measure of the concentration of the dissolved gas particles in the liquid and is a function of the gas pressure. At constant pressure, what. Does Increasing Pressure Increase Solubility.
From techiescientist.com
Factors Affecting Solubility Techiescientist Does Increasing Pressure Increase Solubility Many compounds (such as glucose and ch 3 co 2 na) exhibit a dramatic increase in solubility with increasing temperature. Others (such as nacl and k 2 so 4) exhibit little variation, and. This fundamental principle is crucial in understanding various phenomena, from the fizzing. Describe the effects of temperature and pressure on solubility. At constant pressure, what generally happens. Does Increasing Pressure Increase Solubility.
From www.answersarena.com
[Solved] The solubility of a gas in a liquid increases wit Does Increasing Pressure Increase Solubility Describe the effects of temperature and pressure on solubility. Many compounds (such as glucose and ch 3 co 2 na) exhibit a dramatic increase in solubility with increasing temperature. As you increase the pressure of a gas, the collision frequency. State henry’s law and use it in calculations involving the solubility of a gas in. Others (such as nacl and. Does Increasing Pressure Increase Solubility.
From chemistryhungergames.weebly.com
District 5 Stoichiometry and Solutions 34th Annual Chemistry Hunger Does Increasing Pressure Increase Solubility Many compounds (such as glucose and ch 3 co 2 na) exhibit a dramatic increase in solubility with increasing temperature. The solubility of gases is significantly affected by pressure, as described by henry’s law. Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also.. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID Does Increasing Pressure Increase Solubility The definition of solubility is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature or pressure (in the case of. The solubility of gases is significantly affected by pressure, as described by henry’s law. The solubility is a measure of the concentration of the dissolved gas particles. Does Increasing Pressure Increase Solubility.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Does Increasing Pressure Increase Solubility The definition of solubility is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature or pressure (in the case of. This fundamental principle is crucial in understanding various phenomena, from the fizzing. The solubility is a measure of the concentration of the dissolved gas particles in the. Does Increasing Pressure Increase Solubility.
From slideplayer.com
Solubility of Gases. ppt download Does Increasing Pressure Increase Solubility The solubility of gases is significantly affected by pressure, as described by henry’s law. Others (such as nacl and k 2 so 4) exhibit little variation, and. State henry’s law and use it in calculations involving the solubility of a gas in. This fundamental principle is crucial in understanding various phenomena, from the fizzing. Because the concentration of molecules in. Does Increasing Pressure Increase Solubility.
From slideplayer.com
Solutions How can one differentiate between saturated, unsaturated, and Does Increasing Pressure Increase Solubility Describe the effects of temperature and pressure on solubility. The definition of solubility is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature or pressure (in the case of. This fundamental principle is crucial in understanding various phenomena, from the fizzing. As you increase the pressure of. Does Increasing Pressure Increase Solubility.
From www.numerade.com
SOLVED The solubility of solids increases with increasing pressure Does Increasing Pressure Increase Solubility This fundamental principle is crucial in understanding various phenomena, from the fizzing. Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also. Describe the effects of temperature and pressure on solubility. The solubility is a measure of the concentration of the dissolved gas particles. Does Increasing Pressure Increase Solubility.
From www.vecteezy.com
Sulubility of gases in liquid. As pressure increases, the solubility of Does Increasing Pressure Increase Solubility At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also. Others (such as nacl and k 2 so 4) exhibit little variation, and.. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID5856281 Does Increasing Pressure Increase Solubility This fundamental principle is crucial in understanding various phenomena, from the fizzing. Many compounds (such as glucose and ch 3 co 2 na) exhibit a dramatic increase in solubility with increasing temperature. At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? The solubility is a measure of the. Does Increasing Pressure Increase Solubility.
From edurev.in
Solubility Solutions Class 12 Notes EduRev Does Increasing Pressure Increase Solubility The solubility of gases is significantly affected by pressure, as described by henry’s law. An increase in pressure and an. Increasing pressure increased solubility, but increasing temperature decreases solubility; Describe the effects of temperature and pressure on solubility. The solubility is a measure of the concentration of the dissolved gas particles in the liquid and is a function of the. Does Increasing Pressure Increase Solubility.
From www.scienceabc.com
Why Do Bubbles Form In A Glass Of Water That's Left Out? » ScienceABC Does Increasing Pressure Increase Solubility Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also. Many compounds (such as glucose and ch 3 co 2 na) exhibit a dramatic increase in solubility with increasing temperature. At constant pressure, what generally happens to the solubility of solids and gases when. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Factors Affecting Solubility PowerPoint Presentation, free Does Increasing Pressure Increase Solubility The definition of solubility is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature or pressure (in the case of. An increase in pressure and an. Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the. Does Increasing Pressure Increase Solubility.
From courses.lumenlearning.com
Factors Affecting Solubility Boundless Chemistry Does Increasing Pressure Increase Solubility An increase in pressure and an. Describe the effects of temperature and pressure on solubility. This fundamental principle is crucial in understanding various phenomena, from the fizzing. Others (such as nacl and k 2 so 4) exhibit little variation, and. Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in. Does Increasing Pressure Increase Solubility.
From www.youtube.com
The Effect of Temperature and Pressure on Solubility Mr C YouTube Does Increasing Pressure Increase Solubility Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also. As you increase the pressure of a gas, the collision frequency. This fundamental principle is crucial in understanding various phenomena, from the fizzing. Increasing pressure increased solubility, but increasing temperature decreases solubility; The solubility. Does Increasing Pressure Increase Solubility.
From slideplayer.com
Ch. 16 Solutions. ppt download Does Increasing Pressure Increase Solubility Increasing pressure increased solubility, but increasing temperature decreases solubility; Describe the effects of temperature and pressure on solubility. The solubility is a measure of the concentration of the dissolved gas particles in the liquid and is a function of the gas pressure. As you increase the pressure of a gas, the collision frequency. At constant pressure, what generally happens to. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT 22 Properties of water PowerPoint Presentation ID379006 Does Increasing Pressure Increase Solubility This fundamental principle is crucial in understanding various phenomena, from the fizzing. Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also. At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? Others. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID5798630 Does Increasing Pressure Increase Solubility This fundamental principle is crucial in understanding various phenomena, from the fizzing. Others (such as nacl and k 2 so 4) exhibit little variation, and. Increasing pressure increased solubility, but increasing temperature decreases solubility; Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also.. Does Increasing Pressure Increase Solubility.
From www.researchgate.net
Gassolubility pressure diagram. Download Scientific Diagram Does Increasing Pressure Increase Solubility This fundamental principle is crucial in understanding various phenomena, from the fizzing. Others (such as nacl and k 2 so 4) exhibit little variation, and. Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also. Many compounds (such as glucose and ch 3 co. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT AP Chemistry Unit 2 PowerPoint Presentation, free download ID Does Increasing Pressure Increase Solubility Increasing pressure increased solubility, but increasing temperature decreases solubility; The definition of solubility is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature or pressure (in the case of. Describe the effects of temperature and pressure on solubility. Because the concentration of molecules in the gas phase. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 Does Increasing Pressure Increase Solubility State henry’s law and use it in calculations involving the solubility of a gas in. This fundamental principle is crucial in understanding various phenomena, from the fizzing. The solubility of gases is significantly affected by pressure, as described by henry’s law. Increasing pressure increased solubility, but increasing temperature decreases solubility; The solubility is a measure of the concentration of the. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Chapter 12 Solutions and other complex forces PowerPoint Does Increasing Pressure Increase Solubility State henry’s law and use it in calculations involving the solubility of a gas in. At constant pressure, what generally happens to the solubility of solids and gases when the temperature of a solution is increased? The definition of solubility is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at. Does Increasing Pressure Increase Solubility.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Does Increasing Pressure Increase Solubility The solubility of gases is significantly affected by pressure, as described by henry’s law. Increasing pressure increased solubility, but increasing temperature decreases solubility; State henry’s law and use it in calculations involving the solubility of a gas in. The definition of solubility is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of. Does Increasing Pressure Increase Solubility.