Bromine Reactivity . The robust oxidizing properties of bromine are responsible for many of its chemical reactions. The reaction can be depicted as: It reacts with many metals, sometimes. bromine is a very reactive element. bromine is a dark red liquid that is very reactive and corrosive, and is never found pure in nature. Only one sixth of the. chemical reactions of bromine. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. chemical properties of bromine. bromine generally is much less reactive toward hydrocarbons than chlorine is, both at high temperatures and with activation by. It readily reacts with many elements and compounds, forming bromides. Bromine is highly reactive, similar to other halogens.
from www.examples.com
It reacts with many metals, sometimes. chemical reactions of bromine. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. The reaction can be depicted as: The robust oxidizing properties of bromine are responsible for many of its chemical reactions. It readily reacts with many elements and compounds, forming bromides. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Bromine is highly reactive, similar to other halogens. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −.
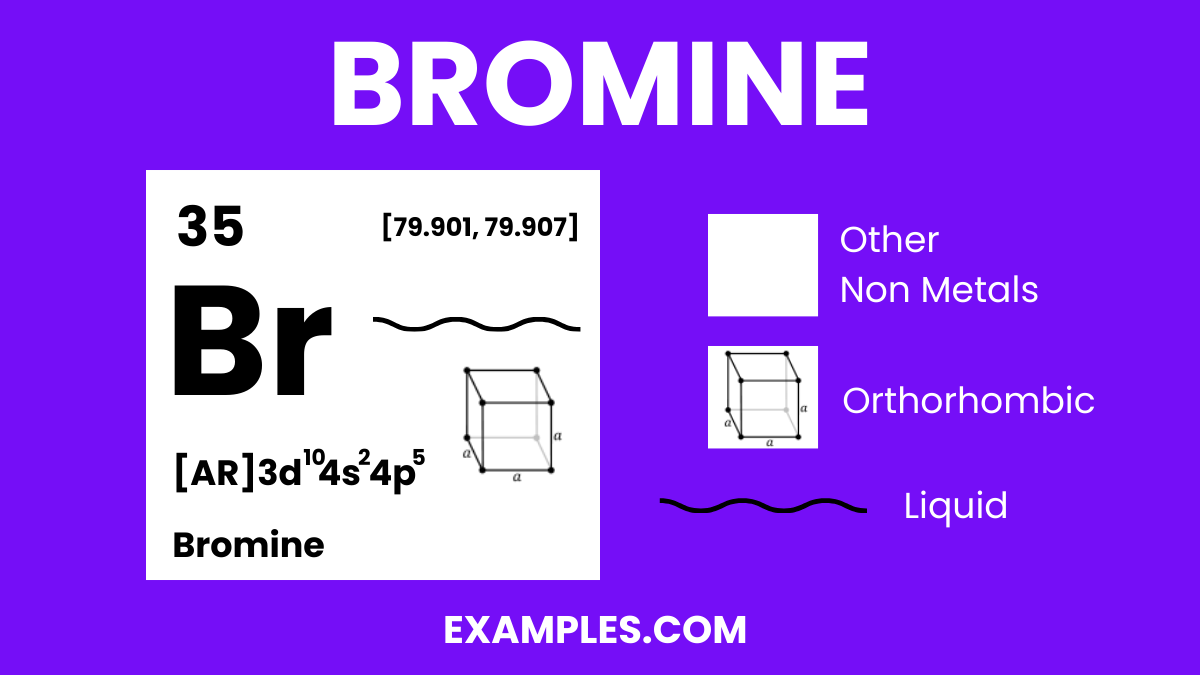
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds
Bromine Reactivity chemical reactions of bromine. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. Only one sixth of the. bromine is a very reactive element. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. bromine is a dark red liquid that is very reactive and corrosive, and is never found pure in nature. chemical reactions of bromine. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. The reaction can be depicted as: It reacts with many metals, sometimes. chemical properties of bromine. bromine generally is much less reactive toward hydrocarbons than chlorine is, both at high temperatures and with activation by. B r 2 + 2. It readily reacts with many elements and compounds, forming bromides. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine Bromine Reactivity chemical properties of bromine. It readily reacts with many elements and compounds, forming bromides. B r 2 + 2. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. bromine is a very reactive element. Bromine is highly reactive, similar to other halogens. bromine generally is. Bromine Reactivity.
From www.youtube.com
Reaction of Aluminium with Bromine YouTube Bromine Reactivity bromine is a dark red liquid that is very reactive and corrosive, and is never found pure in nature. bromine generally is much less reactive toward hydrocarbons than chlorine is, both at high temperatures and with activation by. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. B r 2 + 2. . Bromine Reactivity.
From www.sciencephoto.com
Bromine reaction Stock Image A500/0458 Science Photo Library Bromine Reactivity Bromine is highly reactive, similar to other halogens. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. chemical reactions of bromine. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. bromine is a dark red liquid that is very reactive. Bromine Reactivity.
From www.youtube.com
Reaction of Phenol With Bromine Alcohols, Phenols and Ethers Bromine Reactivity Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. chemical reactions of bromine. bromine is a dark red liquid that is very reactive and corrosive, and is never found pure in nature. While it is less reactive than fluorine or chlorine, it is more reactive than. Bromine Reactivity.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Reactivity Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. B r 2 +. Bromine Reactivity.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation ID Bromine Reactivity chemical reactions of bromine. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. bromine is a very reactive element. It readily reacts with many elements and compounds, forming bromides. It reacts with many metals, sometimes. The reaction can be depicted as: B r 2 + 2. The. Bromine Reactivity.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Bromine Reactivity Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. It readily reacts with many elements and compounds, forming bromides. B r 2 + 2. The reaction can be depicted as: chemical reactions. Bromine Reactivity.
From www.dreamstime.com
Bromine Chemical Stock Photos Free & RoyaltyFree Stock Photos from Bromine Reactivity reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. It reacts with many metals, sometimes. The reaction can be depicted as: It readily reacts with many elements and compounds, forming bromides. chemical reactions of bromine. bromine is a dark red liquid that is very reactive and corrosive,. Bromine Reactivity.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromine vs. Chlorine — Master Bromine Reactivity While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. Bromine is highly reactive, similar to other halogens. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most. Bromine Reactivity.
From www.chemistrysteps.com
Allylic Bromination by NBS with Practice Problems Chemistry Steps Bromine Reactivity Only one sixth of the. It reacts with many metals, sometimes. B r 2 + 2. It readily reacts with many elements and compounds, forming bromides. chemical properties of bromine. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. bromine is a very reactive element. . Bromine Reactivity.
From www.youtube.com
Reaction of Bromine with Phenol YouTube Bromine Reactivity The robust oxidizing properties of bromine are responsible for many of its chemical reactions. It reacts with many metals, sometimes. Bromine is highly reactive, similar to other halogens. The reaction can be depicted as: B r 2 + 2. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. bromine generally is much less. Bromine Reactivity.
From www.youtube.com
Neutralization of Bromine Beautiful Reaction YouTube Bromine Reactivity bromine is a very reactive element. Only one sixth of the. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. bromine is a dark red liquid that is very reactive and corrosive, and. Bromine Reactivity.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Bromine Reactivity While it is less reactive than fluorine or chlorine, it is more reactive than iodine. bromine is a dark red liquid that is very reactive and corrosive, and is never found pure in nature. bromine generally is much less reactive toward hydrocarbons than chlorine is, both at high temperatures and with activation by. Bromine is highly reactive, similar. Bromine Reactivity.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Reactivity Bromine is highly reactive, similar to other halogens. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. The reaction can be depicted as: The robust oxidizing properties of. Bromine Reactivity.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Reactivity Bromine is highly reactive, similar to other halogens. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. chemical reactions of bromine. chemical properties of bromine. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties.. Bromine Reactivity.
From www.chemistrystudent.com
Organic Quick Notes (revision) for A2Level ChemistryStudent Bromine Reactivity Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. It readily reacts with many elements and compounds, forming bromides. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. bromine is a very reactive element. The electron affinity of bromine is intermediate. Bromine Reactivity.
From laurynminowen.blogspot.com
Reaction of Cyclohexene With Bromine LaurynminOwen Bromine Reactivity Bromine is highly reactive, similar to other halogens. bromine is a dark red liquid that is very reactive and corrosive, and is never found pure in nature. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. chemical properties of bromine. chemical reactions of bromine. It. Bromine Reactivity.
From www.researchgate.net
Scheme 3. Reagents and conditions (i) 2bromoacetyl bromide, toluene Bromine Reactivity While it is less reactive than fluorine or chlorine, it is more reactive than iodine. chemical reactions of bromine. It reacts with many metals, sometimes. bromine generally is much less reactive toward hydrocarbons than chlorine is, both at high temperatures and with activation by. Bromine has a high electron affinity, which allows it to accept an electron easily,. Bromine Reactivity.
From www.youtube.com
Reaction of Bromine with Sodium YouTube Bromine Reactivity The reaction can be depicted as: Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. B r 2 + 2. chemical reactions of bromine. bromine is a very reactive element. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. The electron. Bromine Reactivity.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes Master Organic Chemistry Bromine Reactivity bromine is a dark red liquid that is very reactive and corrosive, and is never found pure in nature. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Bromine is highly reactive, similar. Bromine Reactivity.
From www.science-revision.co.uk
Alkene addition reactions Bromine Reactivity Only one sixth of the. bromine is a very reactive element. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. B r 2 + 2. The reaction can be depicted as: Bromine is highly reactive, similar to other halogens. The robust oxidizing properties of bromine are responsible. Bromine Reactivity.
From www.slideserve.com
PPT 7. Alkenes Reactions and Synthesis PowerPoint Presentation ID Bromine Reactivity bromine generally is much less reactive toward hydrocarbons than chlorine is, both at high temperatures and with activation by. It readily reacts with many elements and compounds, forming bromides. chemical properties of bromine. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. Bromine is highly reactive,. Bromine Reactivity.
From www.meritnation.com
In the given image from NCERT textbook , in the equation there is Bromine Reactivity It reacts with many metals, sometimes. B r 2 + 2. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. chemical reactions of bromine. The electron affinity. Bromine Reactivity.
From www.youtube.com
Chemistry experiment 32 Reaction between bromine and aluminium YouTube Bromine Reactivity Only one sixth of the. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. It readily reacts with many elements and compounds, forming bromides. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. The reaction can be depicted as: bromine generally is. Bromine Reactivity.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Reactivity While it is less reactive than fluorine or chlorine, it is more reactive than iodine. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. bromine generally is much less reactive toward hydrocarbons than chlorine is, both at high temperatures and with activation by. bromine is a. Bromine Reactivity.
From www.haikudeck.com
Bromine by Matt Shea Bromine Reactivity chemical reactions of bromine. bromine is a dark red liquid that is very reactive and corrosive, and is never found pure in nature. The robust oxidizing properties of bromine are responsible for many of its chemical reactions. B r 2 + 2. Bromine is highly reactive, similar to other halogens. bromine is a very reactive element. The. Bromine Reactivity.
From www.masterorganicchemistry.com
Bromination of Alkenes Master Organic Chemistry Bromine Reactivity The reaction can be depicted as: bromine is a very reactive element. B r 2 + 2. It readily reacts with many elements and compounds, forming bromides. It reacts with many metals, sometimes. chemical properties of bromine. Bromine is highly reactive, similar to other halogens. bromine generally is much less reactive toward hydrocarbons than chlorine is, both. Bromine Reactivity.
From www.masterorganicchemistry.com
Benzylic Bromination and Benzylic Oxidation Master Organic Chemistry Bromine Reactivity chemical properties of bromine. It readily reacts with many elements and compounds, forming bromides. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. The reaction can be depicted as: Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b. Bromine Reactivity.
From www.youtube.com
Reaction of Sodium (Na) with Bromine (Br2) YouTube Bromine Reactivity It readily reacts with many elements and compounds, forming bromides. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. It reacts with many metals, sometimes. Bromine is highly reactive, similar to other halogens. B r 2 + 2. chemical reactions of bromine. bromine is a very reactive. Bromine Reactivity.
From www.adda247.com
Reactivity series of Metals and Non Metals Bromine Reactivity Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. bromine is a dark red liquid that is very reactive and corrosive, and is never found pure in nature. chemical properties of bromine. The reaction can be depicted as: The electron affinity of bromine is intermediate between. Bromine Reactivity.
From www.youtube.com
Bromine Reactions YouTube Bromine Reactivity The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties. Only one sixth of the. bromine generally is much less reactive toward hydrocarbons than chlorine is, both at high temperatures and with activation by. Bromine has a high electron affinity, which allows it to accept an electron easily,. Bromine Reactivity.
From exouzacfu.blob.core.windows.net
Bromine Of Formula at Delores Humphrey blog Bromine Reactivity Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. B r 2 + 2. It readily reacts with many elements and compounds, forming bromides. bromine generally is much less reactive toward hydrocarbons than chlorine is, both at high temperatures and with activation by. The robust oxidizing properties. Bromine Reactivity.
From www.numerade.com
SOLVED "Draw the mechanism for the bromination reaction.Pyridinium Bromine Reactivity B r 2 + 2. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. bromine is a very reactive element. chemical properties of bromine. chemical. Bromine Reactivity.
From byjus.com
Elimination of bromine from 2 bromobutane results in the formation of Bromine Reactivity chemical properties of bromine. B r 2 + 2. reaction of bromine with bases bromine, br 2 , react with hot aqueous alkali, forming bromate, bro 3 −. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide ion b r −. It reacts with many metals, sometimes. Bromine is highly. Bromine Reactivity.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Bromine Reactivity Bromine is highly reactive, similar to other halogens. It readily reacts with many elements and compounds, forming bromides. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. chemical properties of bromine. B r 2 + 2. Bromine has a high electron affinity, which allows it to accept an electron easily, forming a bromide. Bromine Reactivity.