Which Noble Gas Is Isoelectronic With A Bromide Ion . in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the. isoelectronic | isoelectronic to a particular noble gas |. the ca 2 + ion is therefore isoelectronic with the noble gas ar. the ca 2+ ion is therefore isoelectronic with the noble gas ar. If you look at the electron configuration for bromine, you will. the bromide ion is formed by the addition of one electron. to determine which noble gas is isoelectronic (has the same number of electrons) with each of the given nonmetal. study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon.
from www.chegg.com
the ca 2+ ion is therefore isoelectronic with the noble gas ar. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. the bromide ion is formed by the addition of one electron. If you look at the electron configuration for bromine, you will. isoelectronic | isoelectronic to a particular noble gas |. the ca 2 + ion is therefore isoelectronic with the noble gas ar. to determine which noble gas is isoelectronic (has the same number of electrons) with each of the given nonmetal. study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the.
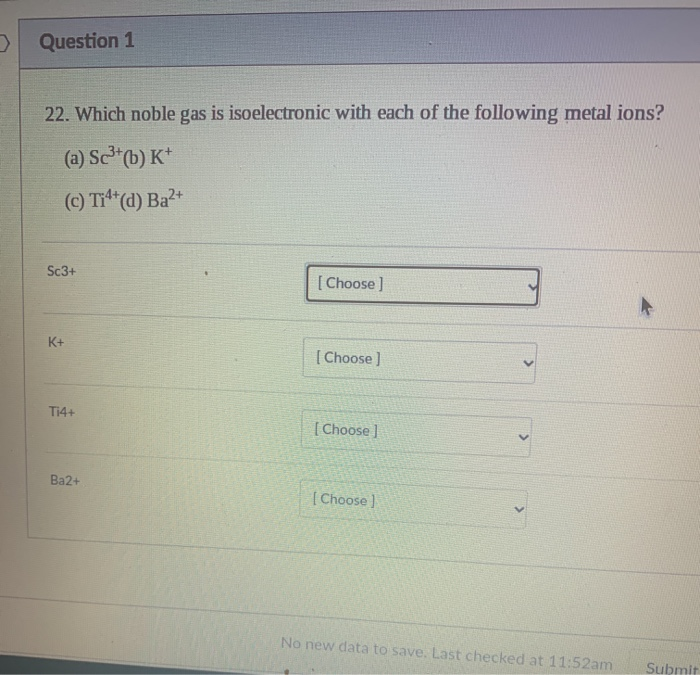
Solved > Question 1 22. Which noble gas is isoelectronic
Which Noble Gas Is Isoelectronic With A Bromide Ion isoelectronic | isoelectronic to a particular noble gas |. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. to determine which noble gas is isoelectronic (has the same number of electrons) with each of the given nonmetal. study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. the bromide ion is formed by the addition of one electron. the ca 2 + ion is therefore isoelectronic with the noble gas ar. in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the. If you look at the electron configuration for bromine, you will. the ca 2+ ion is therefore isoelectronic with the noble gas ar. isoelectronic | isoelectronic to a particular noble gas |.
From www.numerade.com
SOLVED 16. Write the formula and name of the compound formed by these Which Noble Gas Is Isoelectronic With A Bromide Ion looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. the ca 2 + ion is therefore isoelectronic with the noble gas ar. study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. If. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.youtube.com
Isoelectronic Which Ion is isoelectronic to a particular noble gas Which Noble Gas Is Isoelectronic With A Bromide Ion to determine which noble gas is isoelectronic (has the same number of electrons) with each of the given nonmetal. in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the. If you look at the electron configuration for bromine, you will. the ca 2+. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From mungfali.com
Noble Gas Electron Configuration Chart Which Noble Gas Is Isoelectronic With A Bromide Ion the ca 2+ ion is therefore isoelectronic with the noble gas ar. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. isoelectronic | isoelectronic to a particular noble gas |. the ca 2 + ion is therefore isoelectronic with the noble gas ar.. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.numerade.com
SOLVEDRefer to the periodic table and predict which of the following Which Noble Gas Is Isoelectronic With A Bromide Ion isoelectronic | isoelectronic to a particular noble gas |. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. the ca 2 + ion is therefore isoelectronic with the noble gas ar. the ca 2+ ion is therefore isoelectronic with the noble gas ar.. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.numerade.com
SOLVEDWrite the formula and name of the compound formed from the Which Noble Gas Is Isoelectronic With A Bromide Ion the bromide ion is formed by the addition of one electron. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. If you look at the electron configuration for bromine, you will. the ca 2+ ion is therefore isoelectronic with the noble gas ar. . Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.chegg.com
Solved notation Noble box gas notation 20. Construct orbital Which Noble Gas Is Isoelectronic With A Bromide Ion isoelectronic | isoelectronic to a particular noble gas |. study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. the ca 2 + ion is therefore isoelectronic with the noble gas ar. to determine which noble gas is isoelectronic (has the same number of electrons). Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.numerade.com
SOLVED Which noble gas is isoelectronic with the following ions Rb+ Which Noble Gas Is Isoelectronic With A Bromide Ion study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. the ca 2+ ion is therefore isoelectronic with the noble gas ar. in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the.. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From slideplayer.com
GROUP 17 BONDING the HALOGENS ppt download Which Noble Gas Is Isoelectronic With A Bromide Ion isoelectronic | isoelectronic to a particular noble gas |. the ca 2 + ion is therefore isoelectronic with the noble gas ar. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. to determine which noble gas is isoelectronic (has the same number of. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From slideplayer.com
Chemical Bonding Review ppt download Which Noble Gas Is Isoelectronic With A Bromide Ion the ca 2 + ion is therefore isoelectronic with the noble gas ar. the bromide ion is formed by the addition of one electron. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. isoelectronic | isoelectronic to a particular noble gas |. . Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.numerade.com
SOLVED4B 58 C 8B A monatomic ion with charge of +2 has an Which Noble Gas Is Isoelectronic With A Bromide Ion the ca 2 + ion is therefore isoelectronic with the noble gas ar. isoelectronic | isoelectronic to a particular noble gas |. the bromide ion is formed by the addition of one electron. If you look at the electron configuration for bromine, you will. to determine which noble gas is isoelectronic (has the same number of. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From slideplayer.com
Ionic Bonding Formation of IONS. ppt download Which Noble Gas Is Isoelectronic With A Bromide Ion the bromide ion is formed by the addition of one electron. in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the. study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. . Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.showme.com
Noble Gas Electron Configurations Electron Configuration, High School Which Noble Gas Is Isoelectronic With A Bromide Ion If you look at the electron configuration for bromine, you will. study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. in chapter 2,. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From slideplayer.com
Bell Work 9/14/17 Complete Electron Configurations worksheet 14, ppt Which Noble Gas Is Isoelectronic With A Bromide Ion the ca 2 + ion is therefore isoelectronic with the noble gas ar. If you look at the electron configuration for bromine, you will. the ca 2+ ion is therefore isoelectronic with the noble gas ar. in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From courses.lumenlearning.com
Molecular and Ionic Compounds General Chemistry Which Noble Gas Is Isoelectronic With A Bromide Ion in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the. to determine which noble gas is isoelectronic (has the same number of electrons) with each of the given nonmetal. isoelectronic | isoelectronic to a particular noble gas |. the ca 2+ ion. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.slideserve.com
PPT Orbital Diagrams and Electron Configuration PowerPoint Which Noble Gas Is Isoelectronic With A Bromide Ion to determine which noble gas is isoelectronic (has the same number of electrons) with each of the given nonmetal. If you look at the electron configuration for bromine, you will. in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the. isoelectronic | isoelectronic. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.wikihow.com
How to Write a Noble Gas Configuration for Atoms of an Element Which Noble Gas Is Isoelectronic With A Bromide Ion in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the. the ca 2 + ion is therefore isoelectronic with the noble gas ar. If you look at the electron configuration for bromine, you will. isoelectronic | isoelectronic to a particular noble gas |.. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube Which Noble Gas Is Isoelectronic With A Bromide Ion to determine which noble gas is isoelectronic (has the same number of electrons) with each of the given nonmetal. the bromide ion is formed by the addition of one electron. study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. If you look at the electron. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.chegg.com
Solved > Question 1 22. Which noble gas is isoelectronic Which Noble Gas Is Isoelectronic With A Bromide Ion to determine which noble gas is isoelectronic (has the same number of electrons) with each of the given nonmetal. the ca 2+ ion is therefore isoelectronic with the noble gas ar. in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the. isoelectronic. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.chegg.com
Solved Which noble gas ks isoelectronic with each of the Which Noble Gas Is Isoelectronic With A Bromide Ion the ca 2+ ion is therefore isoelectronic with the noble gas ar. in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the. to determine which noble gas is isoelectronic (has the same number of electrons) with each of the given nonmetal. If you. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.numerade.com
Which of the following describes the element Sr Choose all that apply Which Noble Gas Is Isoelectronic With A Bromide Ion If you look at the electron configuration for bromine, you will. isoelectronic | isoelectronic to a particular noble gas |. the ca 2 + ion is therefore isoelectronic with the noble gas ar. the bromide ion is formed by the addition of one electron. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr). Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.chegg.com
Solved 15. Which of the following does not have a noble gas Which Noble Gas Is Isoelectronic With A Bromide Ion the bromide ion is formed by the addition of one electron. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. If you look at the electron configuration for bromine, you will. isoelectronic | isoelectronic to a particular noble gas |. study with quizlet. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.numerade.com
Refer to the periodic table and predict which of the following ions are Which Noble Gas Is Isoelectronic With A Bromide Ion to determine which noble gas is isoelectronic (has the same number of electrons) with each of the given nonmetal. isoelectronic | isoelectronic to a particular noble gas |. in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the. looking at the noble. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.youtube.com
Noble Gas Electron Configurations YouTube Which Noble Gas Is Isoelectronic With A Bromide Ion the bromide ion is formed by the addition of one electron. the ca 2 + ion is therefore isoelectronic with the noble gas ar. study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. If you look at the electron configuration for bromine, you will. . Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.slideserve.com
PPT Orbital Diagrams and Electron Configuration PowerPoint Which Noble Gas Is Isoelectronic With A Bromide Ion the ca 2+ ion is therefore isoelectronic with the noble gas ar. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. the bromide ion is formed by the addition of one electron. the ca 2 + ion is therefore isoelectronic with the noble. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.chegg.com
Solved > Question 1 22. Which noble gas is isoelectronic Which Noble Gas Is Isoelectronic With A Bromide Ion isoelectronic | isoelectronic to a particular noble gas |. the ca 2 + ion is therefore isoelectronic with the noble gas ar. study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. If you look at the electron configuration for bromine, you will. to determine. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.youtube.com
Isoelectronic series and ionic radii YouTube Which Noble Gas Is Isoelectronic With A Bromide Ion to determine which noble gas is isoelectronic (has the same number of electrons) with each of the given nonmetal. the ca 2 + ion is therefore isoelectronic with the noble gas ar. the ca 2+ ion is therefore isoelectronic with the noble gas ar. in chapter 2, we discussed the charges of ions formed for main. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From sciencenotes.org
Noble Gas Configuration Shorthand Electron Configuration Which Noble Gas Is Isoelectronic With A Bromide Ion the bromide ion is formed by the addition of one electron. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. the ca 2+ ion is therefore isoelectronic with the noble gas ar. the ca 2 + ion is therefore isoelectronic with the noble. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From ar.inspiredpencil.com
Orbital Diagram For Bromine Which Noble Gas Is Isoelectronic With A Bromide Ion isoelectronic | isoelectronic to a particular noble gas |. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. If you look at the electron configuration for bromine, you will. study with quizlet and memorize flashcards containing terms like how many electrons must be gained. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.slideshare.net
Unit the periodic table2 Which Noble Gas Is Isoelectronic With A Bromide Ion the ca 2 + ion is therefore isoelectronic with the noble gas ar. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. the bromide ion is formed by the addition of one electron. the ca 2+ ion is therefore isoelectronic with the noble. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From courses.lumenlearning.com
Periodic Variations in Element Properties CHEM 1305 General Which Noble Gas Is Isoelectronic With A Bromide Ion looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36 electrons, xenon (xe) has 54 electrons, and neon. If you look at the electron configuration for bromine, you will. the ca 2 + ion is therefore isoelectronic with the noble gas ar. the ca 2+ ion is therefore isoelectronic with the noble gas. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.numerade.com
SOLVEDWhat is the electron configuration of a bromine atom (You CAN Which Noble Gas Is Isoelectronic With A Bromide Ion study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. the ca 2+ ion is therefore isoelectronic with the noble gas ar. the ca 2 + ion is therefore isoelectronic with the noble gas ar. isoelectronic | isoelectronic to a particular noble gas |. . Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.numerade.com
SOLVED A monatomic ion with a charge of 1 has an electronic Which Noble Gas Is Isoelectronic With A Bromide Ion study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the. If you look at the electron configuration for bromine, you will. isoelectronic. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.numerade.com
SOLVED Give an example of an ionic compound where both the anion and Which Noble Gas Is Isoelectronic With A Bromide Ion to determine which noble gas is isoelectronic (has the same number of electrons) with each of the given nonmetal. the bromide ion is formed by the addition of one electron. the ca 2+ ion is therefore isoelectronic with the noble gas ar. looking at the noble gases, argon (ar) has 18 electrons, krypton (kr) has 36. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.numerade.com
SOLVED Write the electron configurations for the following ions. Tell Which Noble Gas Is Isoelectronic With A Bromide Ion study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. in chapter 2, we discussed the charges of ions formed for main group elements as the gaining or losing of electrons to obtain the. to determine which noble gas is isoelectronic (has the same number of. Which Noble Gas Is Isoelectronic With A Bromide Ion.
From www.youtube.com
Electron Configuration of Ions YouTube Which Noble Gas Is Isoelectronic With A Bromide Ion the bromide ion is formed by the addition of one electron. If you look at the electron configuration for bromine, you will. study with quizlet and memorize flashcards containing terms like how many electrons must be gained by p atom to achieve a. in chapter 2, we discussed the charges of ions formed for main group elements. Which Noble Gas Is Isoelectronic With A Bromide Ion.