Lead Heat Of Combustion . Standard heat of combustion : Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions. The stoichiometry of the reactants. Precise heats of combustion measurements can provide useful information about the structure of molecules. If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. If the heat capacity of the bomb. The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with.
from www.worldatlas.com
The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Precise heats of combustion measurements can provide useful information about the structure of molecules. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. If the heat capacity of the bomb. If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The stoichiometry of the reactants. Standard heat of combustion :
What Are The Properties Of Matter? WorldAtlas
Lead Heat Of Combustion The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. Precise heats of combustion measurements can provide useful information about the structure of molecules. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions. Standard heat of combustion : The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of. If the heat capacity of the bomb. If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. The stoichiometry of the reactants.
From www.studocu.com
step by step EXPERIMENT 1 HEAT OF Lead Heat Of Combustion Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The stoichiometry of the reactants. If the heat capacity of the bomb. Precise heats of combustion measurements can provide useful information about the structure of molecules. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter. Lead Heat Of Combustion.
From www.researchgate.net
(a) The total heat of combustion measured through the experimentations Lead Heat Of Combustion Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. If the heat capacity of the bomb. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard heat of combustion : The heat of combustion is the heat released by a substance. Lead Heat Of Combustion.
From askfilo.com
The heat of combustion of ethene from the given data is CH2 =CH2 ( g)+3O2.. Lead Heat Of Combustion If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. If the heat capacity of the bomb. The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions. Precise heats of combustion. Lead Heat Of Combustion.
From ar.inspiredpencil.com
Heat Of Combustion Graph Lead Heat Of Combustion The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. Precise heats of combustion measurements can provide useful information about the structure of molecules. The heat of combustion is the heat released by a substance when it undergoes. Lead Heat Of Combustion.
From www.studocu.com
Heat of Combustion report Molar Heat of Combustion Experiment Aim Lead Heat Of Combustion The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of. The stoichiometry of the reactants. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during. Lead Heat Of Combustion.
From www.slideserve.com
PPT COMBUSTION REACTIONS PowerPoint Presentation, free download ID Lead Heat Of Combustion Precise heats of combustion measurements can provide useful information about the structure of molecules. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and. Lead Heat Of Combustion.
From www.studocu.com
Experiment No.21 Heat of Combustion alcohol burner Experiment No. 2 Lead Heat Of Combustion The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The stoichiometry of the reactants. Because combustion reactions are exothermic, the temperature. Lead Heat Of Combustion.
From askfilo.com
The heat of combustion of carbon to CO2 is −393.5 kJ/mol. The heat relea.. Lead Heat Of Combustion The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The stoichiometry of the reactants. Precise heats of combustion measurements can provide useful information about the structure of molecules. If the heat capacity. Lead Heat Of Combustion.
From studylib.net
Heat of Combustion Lead Heat Of Combustion The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions. Precise heats of combustion measurements can provide useful information about the structure of molecules. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. If you have. Lead Heat Of Combustion.
From www.numerade.com
SOLVED The heat of combustion of cholesterol, (MC27H46O = 386.46 g.mol Lead Heat Of Combustion The stoichiometry of the reactants. If the heat capacity of the bomb. Precise heats of combustion measurements can provide useful information about the structure of molecules. The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions. If you have a combustion reaction for. Lead Heat Of Combustion.
From www.researchgate.net
Comparison of effective heats of combustion (OC/CDG), complete heats of Lead Heat Of Combustion Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. Standard heat of combustion : The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of. The stoichiometry of the reactants. If the heat capacity of the bomb. If you have. Lead Heat Of Combustion.
From www.grc.nasa.gov
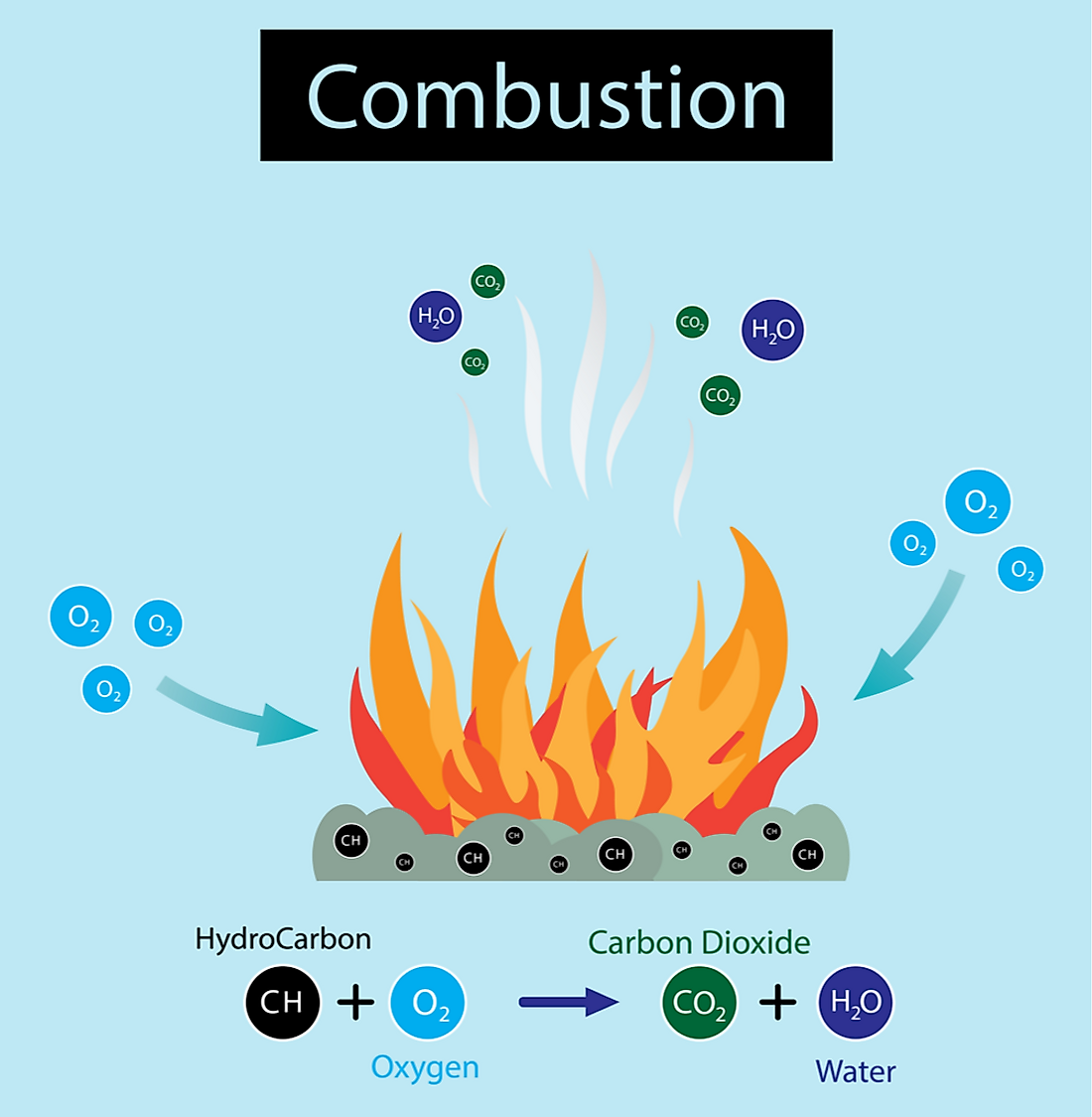
Combustion Lead Heat Of Combustion Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of. If the heat capacity of the bomb. Standard heat of combustion : Precise heats of. Lead Heat Of Combustion.
From www.studocu.com
Experiment 2 Heats of Combustion CHM031L. Chemistry for Engineers Lead Heat Of Combustion Precise heats of combustion measurements can provide useful information about the structure of molecules. The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of. The stoichiometry of the reactants. Standard heat of combustion : The heat of combustion is the heat released by a substance when. Lead Heat Of Combustion.
From www.chegg.com
Solved Combustion (bomb) calorimeter. In an experiment, a Lead Heat Of Combustion The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of. If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. The stoichiometry of the reactants. Standard heat of combustion : If the heat capacity. Lead Heat Of Combustion.
From www.numerade.com
A 0.5865 g sample of lactic acid (HC3H5O3) reacts with oxygen in a Lead Heat Of Combustion If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions. The heating value (or energy value or calorific value) of a. Lead Heat Of Combustion.
From www.dreamstime.com
Solid Fuel Combustion Process Vector Illustration Stock Vector Lead Heat Of Combustion Standard heat of combustion : If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. The. Lead Heat Of Combustion.
From www.youtube.com
The heat of combustion of solid benzoic acid at constant volume is Lead Heat Of Combustion If the heat capacity of the bomb. The stoichiometry of the reactants. Standard heat of combustion : The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. The heating value (or energy value or calorific value) of a. Lead Heat Of Combustion.
From engineeringlearner.com
Types of Combustion Chamber Functions, Advantages & Disadvantages Lead Heat Of Combustion If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Standard enthalpy of combustio n (\. Lead Heat Of Combustion.
From www.chegg.com
Solved Group Combustion Leading Question (read this question Lead Heat Of Combustion Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. The heating value (or energy value. Lead Heat Of Combustion.
From www.engineeringa2z.com
Heat Engine Internal Combustion Engine Engineeringa2z Lead Heat Of Combustion The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. If the heat capacity of the bomb. Standard heat of combustion : The stoichiometry of the reactants. The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions.. Lead Heat Of Combustion.
From studylib.net
Heat of Combustion Lab Lead Heat Of Combustion The stoichiometry of the reactants. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. The heat of combustion is the heat released by a substance when it undergoes complete combustion in. Lead Heat Of Combustion.
From www.coursehero.com
[Solved] Calculate the heat of combustion of methanol, AHo, for 2CH30H Lead Heat Of Combustion If the heat capacity of the bomb. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. The energy liberated when a substance x undergoes complete combustion,. Lead Heat Of Combustion.
From www.researchgate.net
Comparison of parameters of the combustion leading reaction (1 Lead Heat Of Combustion The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. If the heat capacity of the bomb. The stoichiometry of the reactants. Precise heats of combustion measurements can provide useful information about the. Lead Heat Of Combustion.
From www.chegg.com
Solved Given the reactions and overall heats of combustion Lead Heat Of Combustion Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. If the heat capacity of the bomb. The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions. If you have a combustion. Lead Heat Of Combustion.
From www.youtube.com
The heat of combustion of benzene determined in a bomb calorimeter Lead Heat Of Combustion The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of. If the heat capacity of the bomb. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Standard heat of combustion : The stoichiometry of the reactants. Precise heats of. Lead Heat Of Combustion.
From www.coursehero.com
[Solved] Calculate the heat of combustion in kJ 1.0000 mol of ethyl Lead Heat Of Combustion Precise heats of combustion measurements can provide useful information about the structure of molecules. Standard heat of combustion : The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions. The heating value (or energy value or calorific value) of a substance, usually a. Lead Heat Of Combustion.
From www.toppr.com
Heat of combustion of H2(g) = 241.8 kJ mol^1 C(s) = 393.5 kJ mol Lead Heat Of Combustion The stoichiometry of the reactants. Precise heats of combustion measurements can provide useful information about the structure of molecules. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and. Lead Heat Of Combustion.
From spmchemistry.blog.onlinetuition.com.my
Heat of Combustion SPM Chemistry Lead Heat Of Combustion Precise heats of combustion measurements can provide useful information about the structure of molecules. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. If the heat capacity of the bomb. The. Lead Heat Of Combustion.
From www.chegg.com
Solved At constant volume, the heat of combustion of a Lead Heat Of Combustion The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of. If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases. Lead Heat Of Combustion.
From www.worldatlas.com
What Are The Properties Of Matter? WorldAtlas Lead Heat Of Combustion If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. If the heat capacity of the bomb. The heating value (or energy value or calorific value) of a substance, usually a fuel. Lead Heat Of Combustion.
From www.wattalps.com
Thermal runaway propagation and mitigation WATTALPS Advanced Lead Heat Of Combustion Standard heat of combustion : Precise heats of combustion measurements can provide useful information about the structure of molecules. If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. The energy liberated. Lead Heat Of Combustion.
From www.numerade.com
SOLVED The combustion of methanol is shown by the following equation Lead Heat Of Combustion If the heat capacity of the bomb. If you have a combustion reaction for a given species, as discussed in the combustion reactions video, the heat of reaction for. Precise heats of combustion measurements can provide useful information about the structure of molecules. The heat of combustion is the heat released by a substance when it undergoes complete combustion in. Lead Heat Of Combustion.
From www.mdpi.com
Processes Free FullText Improvement of Heat Release Rate Lead Heat Of Combustion Standard heat of combustion : The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of. Precise heats of combustion measurements can provide useful information about the structure of molecules. The heat of combustion is the heat released by a substance when it undergoes complete combustion in. Lead Heat Of Combustion.
From www.numerade.com
SOLVED TABLE 3.1 Heats of Combustion (ΔHc) of Cycloalkanes Heat of Lead Heat Of Combustion The heat of combustion is the heat released by a substance when it undergoes complete combustion in the presence of oxygen at standard temperature and pressure conditions. Because combustion reactions are exothermic, the temperature of the bath and the calorimeter increases during combustion. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Standard. Lead Heat Of Combustion.
From www.semanticscholar.org
Figure 2 from A Review of the Catalytic Effects of Lead‐Based Ballistic Lead Heat Of Combustion If the heat capacity of the bomb. Standard heat of combustion : The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of. The stoichiometry of the reactants. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. The heat of. Lead Heat Of Combustion.