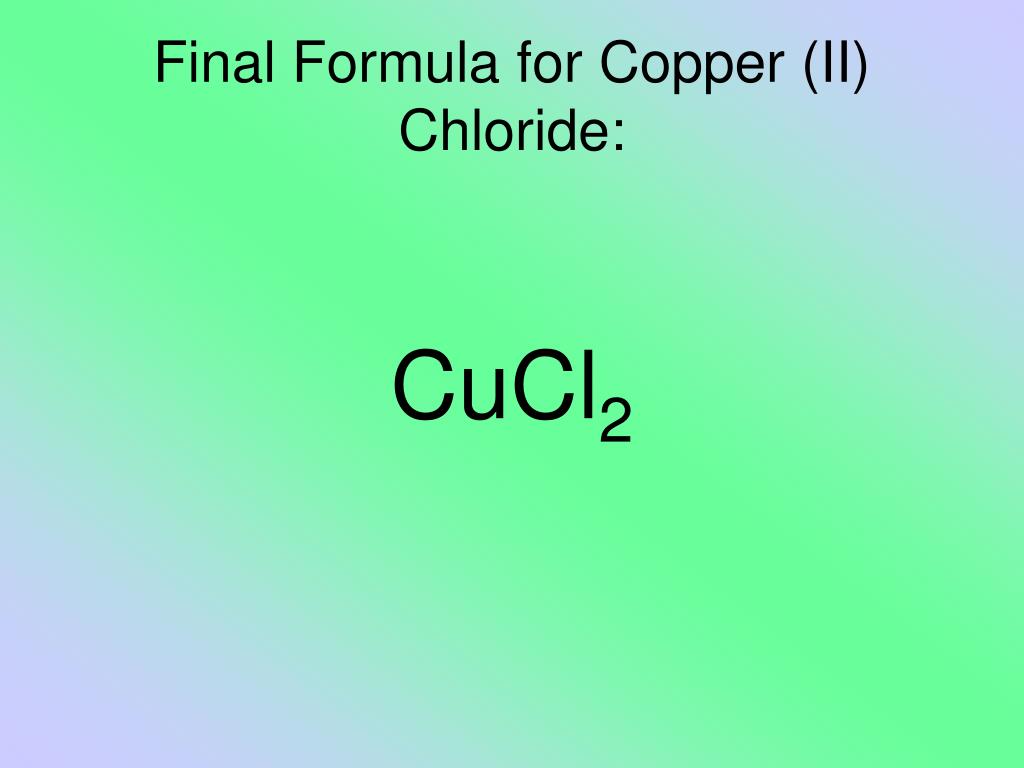
Copper Ii Chloride Melting Point . The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. Copper (ii) chloride is a brown powder that turns red when molten. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. It contains copper in its +2 oxidation. Its chemical formula is cucl 2. Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). Copper(ii) chloride, also known as cupric chloride, is a chemical compound. The boiling point for the anhydrous. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Its melting point is 498 °c.
from www.slideserve.com
Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). Its melting point is 498 °c. Copper(ii) chloride, also known as cupric chloride, is a chemical compound. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. It contains copper in its +2 oxidation. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. Copper (ii) chloride is a brown powder that turns red when molten. The boiling point for the anhydrous. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state.
PPT Naming Compounds and Molecules PowerPoint Presentation, free
Copper Ii Chloride Melting Point Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). The boiling point for the anhydrous. Copper(ii) chloride, also known as cupric chloride, is a chemical compound. Copper (ii) chloride is a brown powder that turns red when molten. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Its melting point is 498 °c. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. It contains copper in its +2 oxidation. Its chemical formula is cucl 2.
From www.slideserve.com
PPT Naming Compounds and Molecules PowerPoint Presentation, free Copper Ii Chloride Melting Point Its melting point is 498 °c. Copper(ii) chloride, also known as cupric chloride, is a chemical compound. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. The boiling point for the anhydrous. The true melting point of 630. Copper Ii Chloride Melting Point.
From www.sciencephoto.com
Copper(II) Chloride Flame Test Stock Image C022/1970 Science Copper Ii Chloride Melting Point The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. The boiling point for the anhydrous. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper(ii) chloride, also known as cupric chloride, is a chemical compound. Copper. Copper Ii Chloride Melting Point.
From www.sciencephoto.com
Copper(II) Chloride Stock Image C027/9485 Science Photo Library Copper Ii Chloride Melting Point Its melting point is 498 °c. Copper (ii) chloride is a brown powder that turns red when molten. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). Copper(ii) chloride, also known as cupric chloride, is a chemical compound.. Copper Ii Chloride Melting Point.
From www.pw.live
Copper II Chloride Formula, Structure, Properties, Uses Copper Ii Chloride Melting Point It contains copper in its +2 oxidation. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. Its chemical formula is cucl 2. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Copper(ii) chloride, also known as cupric chloride, is a chemical. Copper Ii Chloride Melting Point.
From www.youtube.com
Is CuCl2, Copper (II) chloride, Ionic or Covalent? YouTube Copper Ii Chloride Melting Point Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. Copper (ii) chloride is a brown powder that turns red when molten. The boiling point for the anhydrous. The true melting point of 630 °c (1,166 °f). Copper Ii Chloride Melting Point.
From www.sciencemadness.org
Copper(II) chloride Sciencemadness Wiki Copper Ii Chloride Melting Point It contains copper in its +2 oxidation. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. Its chemical formula is cucl 2. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. The exact mass and the. Copper Ii Chloride Melting Point.
From blog.thepipingmart.com
The Melting Point of Copper Wire Explained Copper Ii Chloride Melting Point Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. Its chemical formula is cucl 2. Copper(ii) chloride, also known as cupric chloride, is. Copper Ii Chloride Melting Point.
From pixels.com
Copper (ii) Chloride Solution Photograph by Martyn F. Chillmaid/science Copper Ii Chloride Melting Point The boiling point for the anhydrous. Its chemical formula is cucl 2. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. It contains copper in its +2 oxidation. Copper (ii). Copper Ii Chloride Melting Point.
From paramountchemicals.com.au
Buy Copper (II) Chloride 97 online Paramount Chemicals, Melbourne Copper Ii Chloride Melting Point Copper(ii) chloride, also known as cupric chloride, is a chemical compound. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). Its chemical formula is cucl 2. The boiling point for the anhydrous.. Copper Ii Chloride Melting Point.
From www.tandfonline.com
A copper(II) chloride coordination compound with an adamantanelike Copper Ii Chloride Melting Point It contains copper in its +2 oxidation. The boiling point for the anhydrous. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. Copper(ii) chloride, also known as cupric chloride, is a chemical compound. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Copper (ii) chloride is a brown powder that. Copper Ii Chloride Melting Point.
From www.flinnsci.com
Copper(II) Chloride, Reagent, 100 g Flinn Scientific Copper Ii Chloride Melting Point Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). Copper(ii) chloride, also known as cupric chloride, is a chemical compound. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. The boiling point for the anhydrous. Copper (ii) chloride is a brown. Copper Ii Chloride Melting Point.
From www.slideserve.com
PPT Naming Multivalent Compounds PowerPoint Presentation, free Copper Ii Chloride Melting Point Its chemical formula is cucl 2. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper(ii) chloride, also known as cupric chloride, is a chemical compound. The boiling point for the anhydrous. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. It contains copper in. Copper Ii Chloride Melting Point.
From www.youtube.com
Electrolysis of copper(II) chloride YouTube Copper Ii Chloride Melting Point Its chemical formula is cucl 2. Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). It contains copper in its +2 oxidation. Copper (ii) chloride is a brown powder that turns red when molten. The boiling point for the anhydrous. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting. Copper Ii Chloride Melting Point.
From www.sarchemlabs.com
Copper Chloride Copper II Chloride Sarchem Labs Copper Ii Chloride Melting Point Its melting point is 498 °c. It contains copper in its +2 oxidation. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper (ii) chloride is a brown powder that turns red when molten. The boiling. Copper Ii Chloride Melting Point.
From noahchemicals.com
10 Things to Know About Copper(II) Chloride Noah Chemicals Copper Ii Chloride Melting Point Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. It contains copper in its +2 oxidation. Copper(ii) chloride, also known as cupric chloride, is a chemical compound.. Copper Ii Chloride Melting Point.
From www.showme.com
Melting point and boiling point of copper Science, Physics Copper Ii Chloride Melting Point Its melting point is 498 °c. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. Copper (ii) chloride is a brown powder that turns red when molten. The boiling point for the anhydrous. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. The true melting point of 630 °c (1,166. Copper Ii Chloride Melting Point.
From www.youtube.com
Nails in Copper (II) Chloride (Explained with chemical formulas) YouTube Copper Ii Chloride Melting Point Its chemical formula is cucl 2. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). Copper (ii) chloride is a brown powder that turns red when molten. Its melting point is 498 °c. Copper(ii) chloride is an inorganic chloride of copper in which. Copper Ii Chloride Melting Point.
From www.carolina.com
Copper (II) Chloride, 1.0 M Solution, Anhydrous, Laboratory Grade, 500 Copper Ii Chloride Melting Point It contains copper in its +2 oxidation. Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). Its melting point is 498 °c. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Copper (ii) chloride is a brown powder that turns red when molten. Copper (ii) chloride is hygroscopic and absorbs water in. Copper Ii Chloride Melting Point.
From www.researchgate.net
(PDF) Underglazing with copper(II)chloride Copper Ii Chloride Melting Point The boiling point for the anhydrous. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. Its chemical formula is cucl 2. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper (ii) chloride is hygroscopic and. Copper Ii Chloride Melting Point.
From carbreychem.blogspot.com
Carbrey's Chemistry Blog Copper (II) Chloride and Iron Lab day 1 Copper Ii Chloride Melting Point Copper(ii) chloride, also known as cupric chloride, is a chemical compound. It contains copper in its +2 oxidation. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Its melting point is 498 °c. The true melting. Copper Ii Chloride Melting Point.
From www.sciencephoto.com
Copper(II) Chloride Flame Test Stock Image C022/0777 Science Copper Ii Chloride Melting Point The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. Its melting point is 498. Copper Ii Chloride Melting Point.
From www.sciencephoto.com
Copper(II) chloride dihydrate Stock Image C027/9276 Science Photo Copper Ii Chloride Melting Point Its chemical formula is cucl 2. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. It contains copper in its +2 oxidation. Copper (ii) chloride is a brown powder that turns red. Copper Ii Chloride Melting Point.
From paramountchemicals.com.au
Buy Copper (II) Chloride 97 online Paramount Chemicals, Melbourne Copper Ii Chloride Melting Point Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper (ii) chloride is a brown powder that turns red when molten. Its chemical. Copper Ii Chloride Melting Point.
From www.youtube.com
Burning Copper (II) Chloride In Chemistry Iowa State YouTube Copper Ii Chloride Melting Point Copper (ii) chloride is a brown powder that turns red when molten. It contains copper in its +2 oxidation. The boiling point for the anhydrous. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Its chemical formula is cucl 2. Its melting point is 498 °c. Copper (ii) chloride has a. Copper Ii Chloride Melting Point.
From www.sciencephoto.com
Copper (II) chloride on watch glass Stock Image C053/3347 Science Copper Ii Chloride Melting Point Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. Its melting point is 498 °c. Copper (ii) chloride is a brown powder that turns red when molten.. Copper Ii Chloride Melting Point.
From www.youtube.com
The electrolysis of copper (II) chloride. YouTube Copper Ii Chloride Melting Point It contains copper in its +2 oxidation. Its melting point is 498 °c. The boiling point for the anhydrous. Its chemical formula is cucl 2. Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl. Copper Ii Chloride Melting Point.
From mavink.com
Solder Melting Point Chart Copper Ii Chloride Melting Point It contains copper in its +2 oxidation. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. Copper(ii) chloride, also known as cupric chloride, is a chemical compound. Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). Copper (ii) chloride is a brown powder that turns red when molten. The true. Copper Ii Chloride Melting Point.
From material-properties.org
Copper Thermal Properties Melting Point Thermal Conductivity Copper Ii Chloride Melting Point Its melting point is 498 °c. Copper (ii) chloride is a brown powder that turns red when molten. Its chemical formula is cucl 2. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. The boiling point for the anhydrous. Copper(ii) chloride, also known as cupric chloride, is a chemical compound. Copper. Copper Ii Chloride Melting Point.
From spmscience.blog.onlinetuition.com.my
Electrolysis of Copper (II) Chloride Solution SPM Science Copper Ii Chloride Melting Point Copper (ii) chloride is a brown powder that turns red when molten. Its chemical formula is cucl 2. It contains copper in its +2 oxidation. The boiling point for the anhydrous. Copper(ii) chloride, also known as cupric chloride, is a chemical compound. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. Its melting point. Copper Ii Chloride Melting Point.
From testbook.com
Copper (II) Chloride Formula Structure, Properties and Uses Copper Ii Chloride Melting Point Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). Its chemical formula is cucl 2. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper (ii) chloride is a brown powder that turns red when molten. The true melting point of 630 °c (1,166 °f) can be. Copper Ii Chloride Melting Point.
From www.sciencephoto.com
Copper (II) chloride solution Stock Image C029/5916 Science Photo Copper Ii Chloride Melting Point Copper(ii) chloride, also known as cupric chloride, is a chemical compound. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Its melting point is 498 °c. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. Copper(ii) chloride is an inorganic chloride. Copper Ii Chloride Melting Point.
From www.flinnsci.com
Copper(II) Chloride, Reagent, 500 g Flinn Scientific Copper Ii Chloride Melting Point The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper (ii) chloride is hygroscopic and absorbs water in open air to form the dihydrate,. The. Copper Ii Chloride Melting Point.
From www.researchgate.net
Investigation of the melting point and mass change of coppercontaining Copper Ii Chloride Melting Point Its melting point is 498 °c. The exact mass and the monoisotopic mass of cupric chloride is 132.867 g/mol. Copper(ii) chloride, also known as cupric chloride, is a chemical compound. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. The true melting point of 630 °c (1,166 °f) can be extrapolated. Copper Ii Chloride Melting Point.
From www.sciencephoto.com
Copper(II) Chloride Flame Test Stock Image C022/1972 Science Copper Ii Chloride Melting Point Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). It contains copper in its +2 oxidation. The boiling point for the anhydrous. The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. Copper (ii) chloride is hygroscopic and absorbs water in open. Copper Ii Chloride Melting Point.
From www.carolina.com
Copper (II) Chloride, 0.1 M Solution, Anhydrous, Laboratory Grade, 500 Copper Ii Chloride Melting Point Copper (ii) chloride has a melting point of 498°c (anhydrous) or 100°c (dihydrate). The true melting point of 630 °c (1,166 °f) can be extrapolated by using the melting points of the mixtures of cucl and cucl2. It contains copper in its +2 oxidation. Its chemical formula is cucl 2. The boiling point for the anhydrous. Its melting point is. Copper Ii Chloride Melting Point.