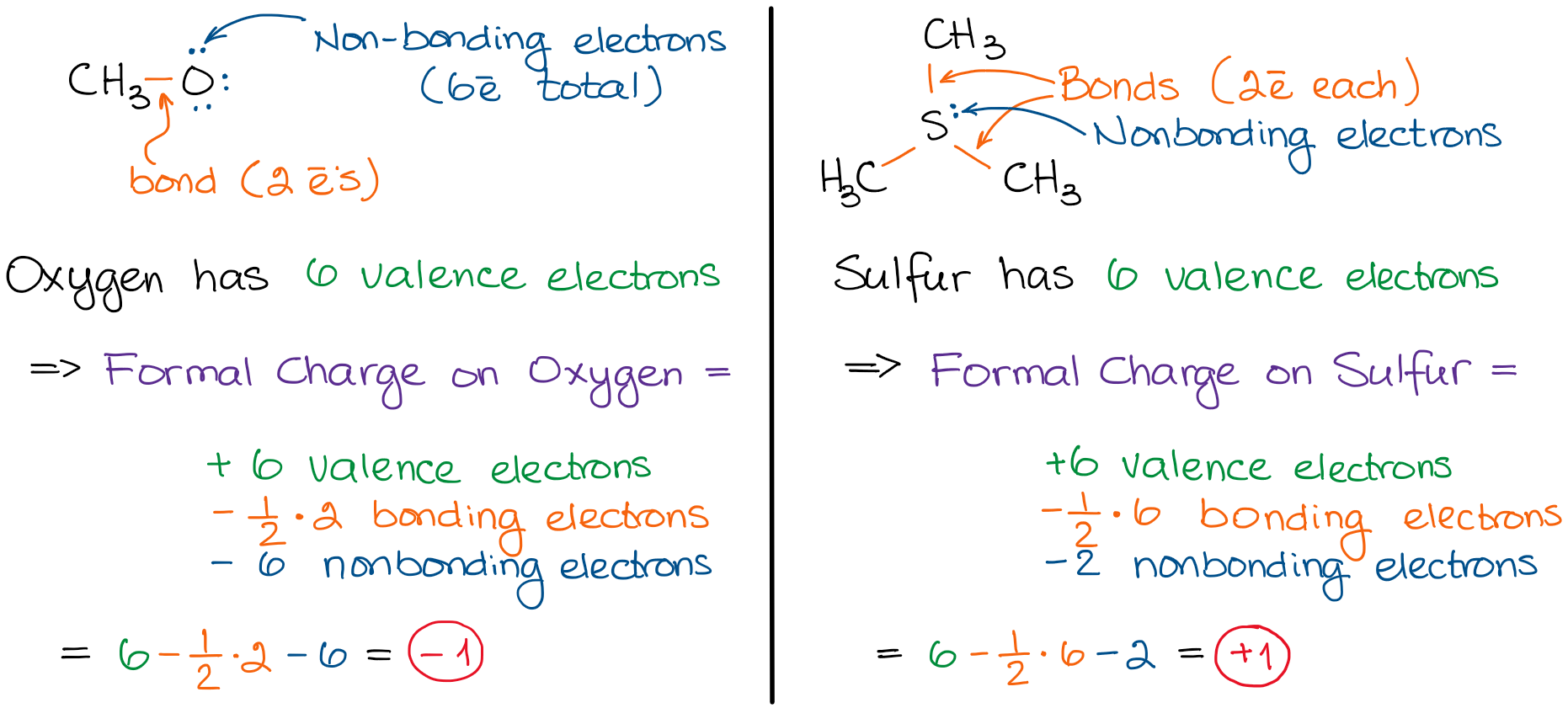
Mg Formal Charge . To calculate the formal charge of an atom, we start by: Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. How to calculate formal charge: The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on other atoms present in the molecule. But more on that later! Evaluating the number of valence. How to calculate formal charge. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the ways electrons are actually shared between atoms in a bond. But few (students or tutors) go on to. The mg thus does not have a filled 3s/3p valence shell, which would be eight. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally.
from www.organicchemistrytutor.com
But more on that later! This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the ways electrons are actually shared between atoms in a bond. The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on other atoms present in the molecule. How to calculate formal charge: Evaluating the number of valence. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. But few (students or tutors) go on to. To calculate the formal charge of an atom, we start by: How to calculate formal charge. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally.
Formal Charges — Organic Chemistry Tutor
Mg Formal Charge But more on that later! The mg thus does not have a filled 3s/3p valence shell, which would be eight. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. To calculate the formal charge of an atom, we start by: Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. How to calculate formal charge: Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the ways electrons are actually shared between atoms in a bond. Evaluating the number of valence. The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on other atoms present in the molecule. But few (students or tutors) go on to. But more on that later! How to calculate formal charge.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry Mg Formal Charge A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. How to calculate formal charge. The mg thus does not have a filled 3s/3p valence shell, which would be eight. But few (students or tutors) go on to. To calculate the formal charge of an. Mg Formal Charge.
From study.com
Formal Charge Formula How to Calculate Formal Charge Video & Lesson Mg Formal Charge How to calculate formal charge. The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on other atoms present in the molecule. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. This is a hypothetical. Mg Formal Charge.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry Mg Formal Charge How to calculate formal charge: This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the ways electrons are actually shared between atoms in a bond. How to calculate formal charge. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared. Mg Formal Charge.
From www.organicchemistrytutor.com
Formal Charges — Organic Chemistry Tutor Mg Formal Charge This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the ways electrons are actually shared between atoms in a bond. But more on that later! How to calculate formal charge. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared. Mg Formal Charge.
From www.slideserve.com
PPT Formal Charge PowerPoint Presentation, free download ID3870031 Mg Formal Charge The mg thus does not have a filled 3s/3p valence shell, which would be eight. To calculate the formal charge of an atom, we start by: Evaluating the number of valence. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. But more on that later! Formal charge is assigned. Mg Formal Charge.
From chemisfast.blogspot.com
Formal charge, formal charge calculation and significance of formal Mg Formal Charge How to calculate formal charge. To calculate the formal charge of an atom, we start by: But more on that later! But few (students or tutors) go on to. The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on other atoms present in the molecule. Formal charge. Mg Formal Charge.
From www.pathwaystochemistry.com
Formal Charge Pathways to Chemistry Mg Formal Charge But more on that later! How to calculate formal charge. Evaluating the number of valence. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. The mg thus does not have a filled 3s/3p valence shell, which would be eight. But few (students or tutors) go on to.. Mg Formal Charge.
From www.collegesearch.in
Formal Charge Formula Definitions, Examples, Significance, Fun Facts Mg Formal Charge How to calculate formal charge: But more on that later! To calculate the formal charge of an atom, we start by: Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. This is a hypothetical measure, not a real representation of the actual charge on an atom, which. Mg Formal Charge.
From www.bartleby.com
Formal Charges bartleby Mg Formal Charge But more on that later! A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. To calculate the formal charge of an atom, we start by: The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it. Mg Formal Charge.
From general.chemistrysteps.com
Formal Charges Chemistry Steps Mg Formal Charge The mg thus does not have a filled 3s/3p valence shell, which would be eight. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. To calculate the formal charge of an atom, we start by: This is a hypothetical measure, not a real representation. Mg Formal Charge.
From www.animalia-life.club
Charge Formula Mg Formal Charge Evaluating the number of valence. How to calculate formal charge: But more on that later! The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on other atoms present in the molecule. The mg thus does not have a filled 3s/3p valence shell, which would be eight. But. Mg Formal Charge.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry Mg Formal Charge To calculate the formal charge of an atom, we start by: Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. But more on that later! This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the ways electrons are actually. Mg Formal Charge.
From www.chemistrywithlaura.com
Formal charge practice problems with answers (PDF) Mg Formal Charge How to calculate formal charge. But few (students or tutors) go on to. Evaluating the number of valence. How to calculate formal charge: But more on that later! Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. The term “formal” means that this charge is not necessarily on the. Mg Formal Charge.
From www.slideserve.com
PPT Formal Charge PowerPoint Presentation, free download ID3870031 Mg Formal Charge But more on that later! But few (students or tutors) go on to. Evaluating the number of valence. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical. Mg Formal Charge.
From www.youtube.com
Formal Charge YouTube Mg Formal Charge Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. The mg thus does not have a filled 3s/3p valence shell, which would be eight. The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on. Mg Formal Charge.
From www.youtube.com
How to calculate the formal charge quickly YouTube Mg Formal Charge Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. But more on that later! To calculate the formal charge of an atom, we start by: But few. Mg Formal Charge.
From www.masterorganicchemistry.com
How To Calculate Formal Charge Mg Formal Charge A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the ways electrons are actually shared between atoms in a bond. How to calculate formal charge:. Mg Formal Charge.
From www.mounthnails.com
Formal Charge A Comprehensive Guide for Chemistry Students Mg Formal Charge Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. But few (students or tutors) go on to. But more on that later! How to calculate formal charge. The mg thus does not have a filled 3s/3p valence shell, which would be eight. Evaluating the number of valence. The term. Mg Formal Charge.
From www.masterorganicchemistry.com
How To Calculate Formal Charge Mg Formal Charge How to calculate formal charge. But few (students or tutors) go on to. To calculate the formal charge of an atom, we start by: The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on other atoms present in the molecule. Formal charge is a charge assigned to. Mg Formal Charge.
From www.organicchemistrytutor.com
Formal Charges — Organic Chemistry Tutor Mg Formal Charge But few (students or tutors) go on to. How to calculate formal charge: How to calculate formal charge. The mg thus does not have a filled 3s/3p valence shell, which would be eight. But more on that later! Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. Evaluating the. Mg Formal Charge.
From chem.libretexts.org
Formal Charge Chemistry LibreTexts Mg Formal Charge This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the ways electrons are actually shared between atoms in a bond. Evaluating the number of valence. The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on other atoms. Mg Formal Charge.
From www.makingmolecules.com
Formal Charges — Making Molecules Mg Formal Charge The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on other atoms present in the molecule. But few (students or tutors) go on to. How to calculate formal charge. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in. Mg Formal Charge.
From www.slideserve.com
PPT FORMAL CHARGE PowerPoint Presentation, free download ID4196511 Mg Formal Charge Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. How to calculate formal charge. This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the ways electrons are actually shared between atoms in a bond. The term “formal”. Mg Formal Charge.
From www.slideserve.com
PPT Lesson 4 Methanol PowerPoint Presentation, free download ID Mg Formal Charge A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. But few (students or tutors) go on to. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. This is a hypothetical measure, not a. Mg Formal Charge.
From www.slideserve.com
PPT Formal Charge PowerPoint Presentation, free download ID338614 Mg Formal Charge Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. But few (students or tutors) go on to. How to calculate formal charge. The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on other atoms present in. Mg Formal Charge.
From www.makingmolecules.com
Formal Charges — Making Molecules Mg Formal Charge Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks. Mg Formal Charge.
From www.youtube.com
Calculating NO3 Formal Charges Calculating Formal Charges for NO3 Mg Formal Charge A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. How to calculate formal charge. How to calculate formal charge: Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. This is a. Mg Formal Charge.
From www.collegesearch.in
Formal Charge Formula Definitions, Examples, Significance, Fun Facts Mg Formal Charge Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. To calculate the formal charge of an atom, we start by: The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on other atoms present in. Mg Formal Charge.
From wisc.pb.unizin.org
M8Q3 Resonance Structures and Formal Charge Chem 103/104 Resource Book Mg Formal Charge How to calculate formal charge. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. But more on that later! The term “formal” means that this charge is. Mg Formal Charge.
From www.collegesearch.in
Formal Charge Formula Definitions, Examples, Significance, Fun Facts Mg Formal Charge A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. But few (students or tutors) go on to. But more on that later!. Mg Formal Charge.
From www.youtube.com
How to Calculate the Formal Charges for ClO2 (Chlorite ion) YouTube Mg Formal Charge Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. But few (students or tutors) go on to. But more on that later! Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. The mg thus does not. Mg Formal Charge.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation, free download ID4438864 Mg Formal Charge This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the ways electrons are actually shared between atoms in a bond. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. To calculate the formal charge of an atom, we start. Mg Formal Charge.
From www.organicchemistrytutor.com
Formal Charges — Organic Chemistry Tutor Mg Formal Charge Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. How to calculate formal charge: Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. This is a hypothetical measure, not a real representation of the actual charge. Mg Formal Charge.
From www.expii.com
Formal Charge — Overview & Calculation Expii Mg Formal Charge How to calculate formal charge: This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the ways electrons are actually shared between atoms in a bond. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. But more on that later!. Mg Formal Charge.
From mavink.com
Formal Charge Lewis Structure Mg Formal Charge To calculate the formal charge of an atom, we start by: The term “formal” means that this charge is not necessarily on the presented atom because in some cases, it is also prevalent on other atoms present in the molecule. This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the. Mg Formal Charge.