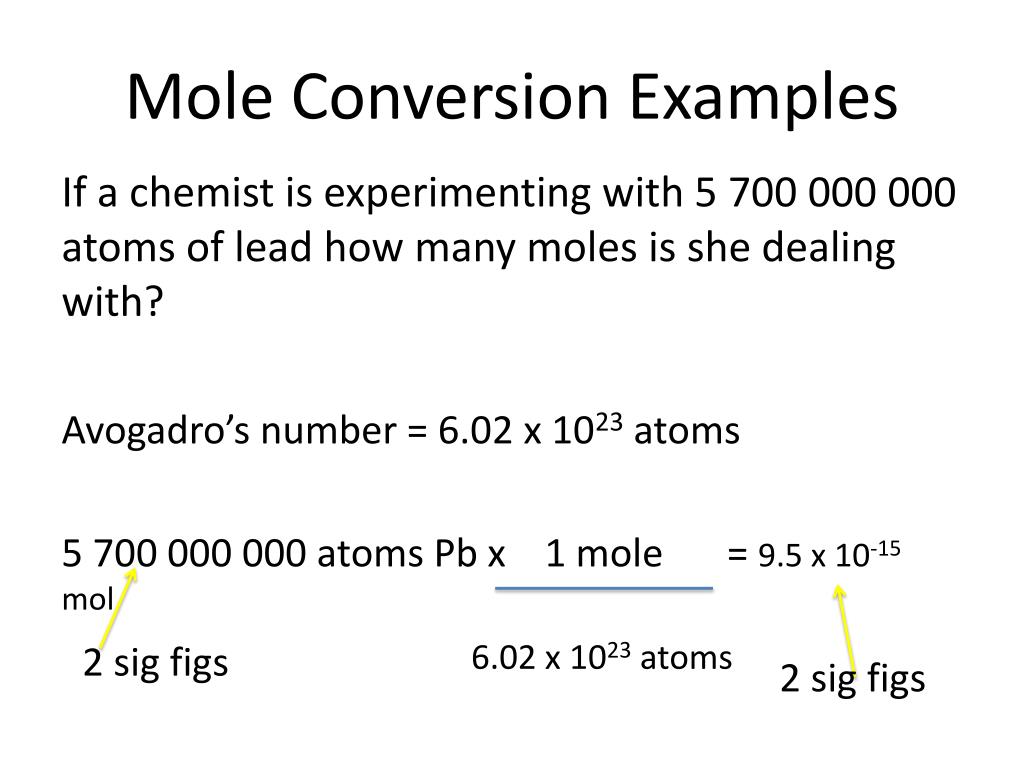
Types Of Mole Conversions . Perform conversions between mass and moles of a substance. One mole is equal to 6.022 x 10^23 atoms,. Moles are a type of unit conversion used in chemistry to measure the amount of substances. If you have 22.414 grams. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Determine the mass of 0.752 mol of h 2 gas. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick.
from www.slideserve.com
How many moles of molecular hydrogen are present in 6.022 grams of h 2? If you have 22.414 grams. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Determine the mass of 0.752 mol of h 2 gas. Moles are a type of unit conversion used in chemistry to measure the amount of substances. One mole is equal to 6.022 x 10^23 atoms,. Perform conversions between mass and moles of a substance. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in.
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free
Types Of Mole Conversions Moles are a type of unit conversion used in chemistry to measure the amount of substances. If you have 22.414 grams. Perform conversions between mass and moles of a substance. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Determine the mass of 0.752 mol of h 2 gas. One mole is equal to 6.022 x 10^23 atoms,. Moles are a type of unit conversion used in chemistry to measure the amount of substances.
From www.studypool.com
SOLUTION Mole conversion diagram Studypool Types Of Mole Conversions Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Moles are a type of unit conversion used in chemistry to measure the amount of substances. Perform conversions between mass and. Types Of Mole Conversions.
From www.showme.com
ShowMe mole conversion Types Of Mole Conversions Moles are a type of unit conversion used in chemistry to measure the amount of substances. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in. Whether you’re converting from moles to grams, moles. Types Of Mole Conversions.
From www.pinterest.com
Mole Conversions Made Easy How to Convert Between Grams and Moles Types Of Mole Conversions Determine the mass of 0.752 mol of h 2 gas. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Perform conversions between mass and moles of a substance. If you have 22.414 grams. Moles are a type of unit conversion used in chemistry to measure the amount of substances. Convert from mass or moles of. Types Of Mole Conversions.
From wou.edu
Chapter 6 Quantities in Chemical Reactions Chemistry Types Of Mole Conversions Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in. Moles are a type of unit conversion used in chemistry to measure the amount of substances. Whether you’re converting from moles to grams, moles. Types Of Mole Conversions.
From mungfali.com
Mole Conversion Cheat Sheet Types Of Mole Conversions Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. How many moles of. Types Of Mole Conversions.
From www.slideserve.com
PPT Mole conversions PowerPoint Presentation, free download ID5595247 Types Of Mole Conversions If you have 22.414 grams. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Perform conversions between mass and moles of a substance. Moles are a type of unit conversion used in chemistry to measure the amount of substances. Previously, you learned to balance chemical equations by comparing the numbers. Types Of Mole Conversions.
From chemistrymysteries.blogspot.com
Chemistry Mysteries Mole Conversions Types Of Mole Conversions Moles are a type of unit conversion used in chemistry to measure the amount of substances. If you have 22.414 grams. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in. One mole. Types Of Mole Conversions.
From www.slideserve.com
PPT The mole PowerPoint Presentation, free download ID4135911 Types Of Mole Conversions How many moles of molecular hydrogen are present in 6.022 grams of h 2? Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. One mole is equal to 6.022 x 10^23 atoms,. Perform conversions between mass and moles of a substance. Whether you’re converting from moles to grams, moles to. Types Of Mole Conversions.
From www.sophia.org
Mole Conversions Dimensional Analysis Tutorial Sophia Learning Types Of Mole Conversions Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Determine the mass of 0.752 mol of h 2 gas. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. How many moles of molecular hydrogen are present in 6.022 grams. Types Of Mole Conversions.
From www.slideserve.com
PPT X Chemistry Unit 8 The Mole PowerPoint Presentation, free Types Of Mole Conversions How many moles of molecular hydrogen are present in 6.022 grams of h 2? Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Determine the mass of 0.752 mol of h 2 gas. One mole is equal to 6.022 x 10^23 atoms,. If you have 22.414 grams. Perform conversions. Types Of Mole Conversions.
From thechemguys.blogspot.com
Chemestry 11 Lessons Mole Conversions Types Of Mole Conversions One mole is equal to 6.022 x 10^23 atoms,. If you have 22.414 grams. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Perform conversions between mass and moles of a substance. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Moles are a type. Types Of Mole Conversions.
From sciencenotes.org
Moles to Grams Conversion Examples Types Of Mole Conversions Perform conversions between mass and moles of a substance. Moles are a type of unit conversion used in chemistry to measure the amount of substances. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Determine the mass of 0.752 mol of h 2 gas. Convert from mass or moles. Types Of Mole Conversions.
From sscchemistry.weebly.com
Mole Conversions and Calculation SSC Chemistry Types Of Mole Conversions Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Moles are a type of unit conversion used in chemistry to measure the amount of substances. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in. One mole is equal to 6.022 x. Types Of Mole Conversions.
From solutionstoichiometry.weebly.com
Conversions Solution Stoichiometry Types Of Mole Conversions One mole is equal to 6.022 x 10^23 atoms,. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. If you have 22.414 grams. Determine the mass of 0.752 mol of h 2 gas. Moles are a type of unit conversion used in chemistry to measure the amount of substances. Previously,. Types Of Mole Conversions.
From general.chemistrysteps.com
How To Convert Grams To Moles Chemistry Steps Types Of Mole Conversions One mole is equal to 6.022 x 10^23 atoms,. Perform conversions between mass and moles of a substance. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Determine the mass of 0.752 mol of h 2. Types Of Mole Conversions.
From www.showme.com
1Step Mole Conversions Science, Chemistry, Molecules, Conversions Types Of Mole Conversions How many moles of molecular hydrogen are present in 6.022 grams of h 2? If you have 22.414 grams. Determine the mass of 0.752 mol of h 2 gas. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Previously, you learned to balance chemical equations by comparing the numbers. Types Of Mole Conversions.
From www.youtube.com
How to Convert Between Mass and Moles (Mole Conversion Part 1) YouTube Types Of Mole Conversions Determine the mass of 0.752 mol of h 2 gas. Moles are a type of unit conversion used in chemistry to measure the amount of substances. If you have 22.414 grams. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Convert from mass or moles of one substance to mass or moles of another substance. Types Of Mole Conversions.
From www.youtube.com
Mole Conversions Tutorial how to convert mole mass, mole particle Types Of Mole Conversions Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Moles are a type of unit conversion used in chemistry to measure the amount of substances. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in. Whether you’re converting from moles to grams, moles. Types Of Mole Conversions.
From www.slideserve.com
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free Types Of Mole Conversions Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. How many moles of molecular hydrogen are present in 6.022 grams of h 2? If you have 22.414 grams. Perform conversions. Types Of Mole Conversions.
From www.templateroller.com
Chemistry Cheat Sheet Mole Conversion Chart Download Printable PDF Types Of Mole Conversions Determine the mass of 0.752 mol of h 2 gas. Moles are a type of unit conversion used in chemistry to measure the amount of substances. How many moles of molecular hydrogen are present in 6.022 grams of h 2? If you have 22.414 grams. Previously, you learned to balance chemical equations by comparing the numbers of each type of. Types Of Mole Conversions.
From www.expii.com
Mole Road Map — Overview & Examples of Conversions Expii Types Of Mole Conversions Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in. One mole is equal to 6.022 x 10^23 atoms,. Determine the mass of 0.752 mol of h 2 gas. Perform conversions between mass. Types Of Mole Conversions.
From www.showme.com
Basic mole conversions part 1 Science, Chemistry, Moles ShowMe Types Of Mole Conversions Moles are a type of unit conversion used in chemistry to measure the amount of substances. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Perform conversions between mass and moles of a substance. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Convert. Types Of Mole Conversions.
From www.youtube.com
Mole Mole Conversion Mole Mass Conversion Mass Mass Conversion Types Of Mole Conversions If you have 22.414 grams. How many moles of molecular hydrogen are present in 6.022 grams of h 2? One mole is equal to 6.022 x 10^23 atoms,. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Previously, you learned to balance chemical equations by comparing the numbers of each. Types Of Mole Conversions.
From www.pinterest.com
Mole conversion chart Teaching chemistry, Chemistry classroom Types Of Mole Conversions If you have 22.414 grams. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Moles are a type of unit conversion used in chemistry to measure the amount of substances. One mole is equal to. Types Of Mole Conversions.
From www.slideserve.com
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free Types Of Mole Conversions Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Moles are a type of unit conversion used in chemistry to measure the amount of substances. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. One mole is equal to. Types Of Mole Conversions.
From www.showme.com
1 step vs. 2 step conversions Science, Chemistry, Moles To Mass Types Of Mole Conversions Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. One mole is equal to 6.022 x 10^23 atoms,. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. How many moles of molecular hydrogen are present in 6.022 grams of. Types Of Mole Conversions.
From www.slideserve.com
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free Types Of Mole Conversions One mole is equal to 6.022 x 10^23 atoms,. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Moles are a type of unit conversion used in chemistry to measure the amount of substances. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in. Perform conversions between mass. Types Of Mole Conversions.
From www.slideserve.com
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free Types Of Mole Conversions Perform conversions between mass and moles of a substance. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Moles are a type of unit conversion used in chemistry to measure the amount of. Types Of Mole Conversions.
From www.pinterest.co.uk
A mole conversion is when the mole is able the measure both mass and Types Of Mole Conversions How many moles of molecular hydrogen are present in 6.022 grams of h 2? Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. One mole is equal to 6.022 x 10^23 atoms,. Determine the mass of 0.752 mol of h 2 gas. Perform conversions between mass and moles of a. Types Of Mole Conversions.
From www.slideserve.com
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free Types Of Mole Conversions How many moles of molecular hydrogen are present in 6.022 grams of h 2? One mole is equal to 6.022 x 10^23 atoms,. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in. Whether. Types Of Mole Conversions.
From screenpal.com
mole conversion tutoring Types Of Mole Conversions Moles are a type of unit conversion used in chemistry to measure the amount of substances. One mole is equal to 6.022 x 10^23 atoms,. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Perform conversions. Types Of Mole Conversions.
From www.slideserve.com
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free Types Of Mole Conversions Perform conversions between mass and moles of a substance. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Moles are a type of unit conversion used in chemistry to measure the amount of substances. If. Types Of Mole Conversions.
From classdbdaecher.z13.web.core.windows.net
Two Step Mole Conversions Types Of Mole Conversions Determine the mass of 0.752 mol of h 2 gas. Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick. Perform conversions between mass and moles of a substance. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Previously, you. Types Of Mole Conversions.
From classhoffmann.z19.web.core.windows.net
Chemistry Conversion Chart Moles Types Of Mole Conversions If you have 22.414 grams. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Moles are a type of unit conversion used in chemistry to measure the amount of substances. Determine the mass of 0.752 mol of h 2 gas. Perform conversions between mass and moles of a substance. Previously, you learned to balance chemical. Types Of Mole Conversions.
From www.slideserve.com
PPT The Mole PowerPoint Presentation, free download ID16795 Types Of Mole Conversions How many moles of molecular hydrogen are present in 6.022 grams of h 2? Determine the mass of 0.752 mol of h 2 gas. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in.. Types Of Mole Conversions.