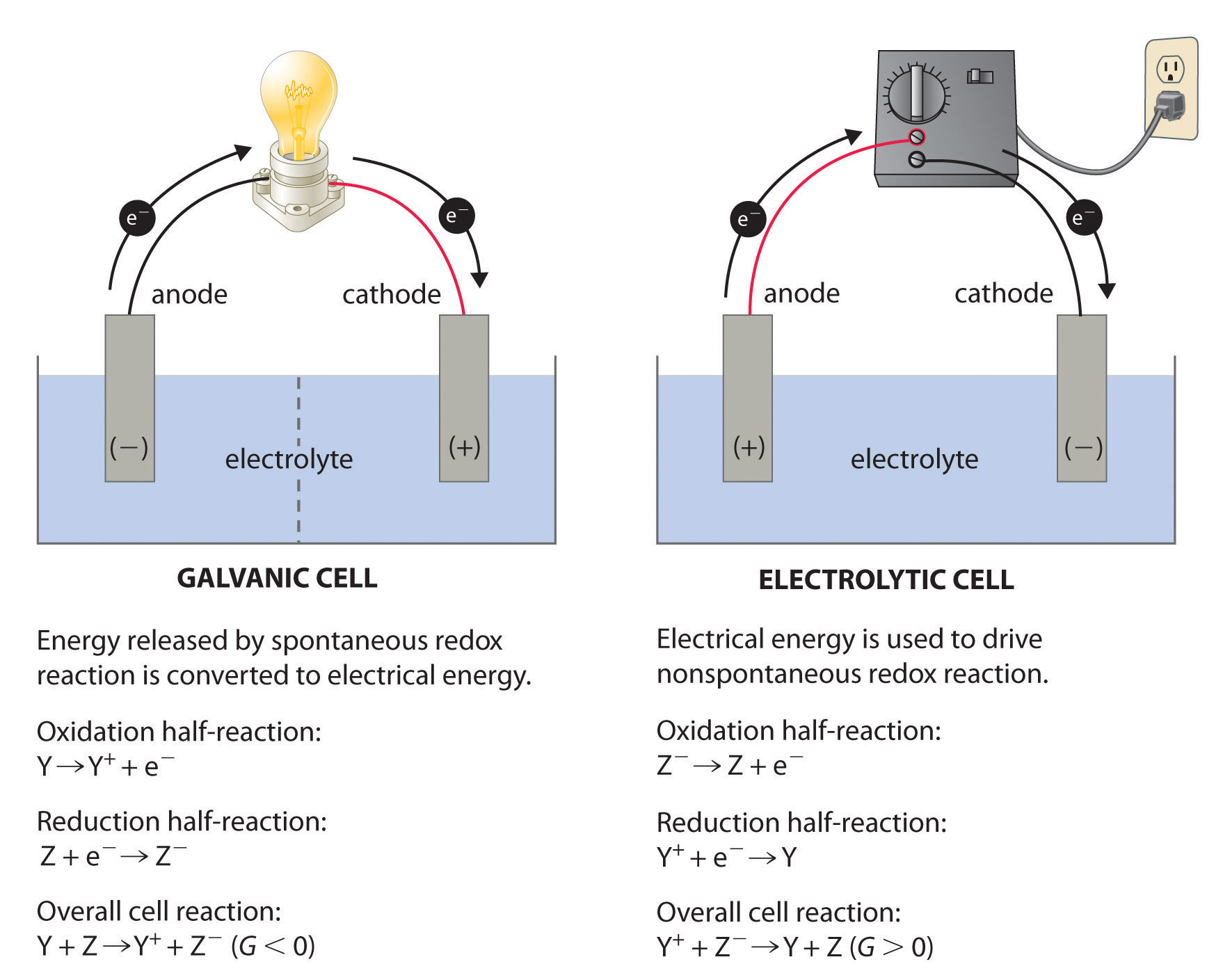
Standard Hydrogen Electrode Acts As Both Anode And Cathode Why . The value of the standard electrode potential is zero, which forms. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The cell potential of an electrochemical cell is the difference between its cathode and anode. When a standard hydrogen electrode undergoes. It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. This is because it acts as a reference for comparison with any other electrode. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. Standard hydrogen electrode can work both as an anode and as a cathode.
from rohanfersmorrison.blogspot.com
Examples of using the standard hydrogen electrode to determine reduction potentials are given. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The structure of the standard hydrogen electrode is described. It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. This is because it acts as a reference for comparison with any other electrode. Standard hydrogen electrode can work both as an anode and as a cathode. The value of the standard electrode potential is zero, which forms. When a standard hydrogen electrode undergoes. The cell potential of an electrochemical cell is the difference between its cathode and anode.
Identify the Conditions for a Standard Electrochemical Cell.
Standard Hydrogen Electrode Acts As Both Anode And Cathode Why This is because it acts as a reference for comparison with any other electrode. It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. This is because it acts as a reference for comparison with any other electrode. Standard hydrogen electrode can work both as an anode and as a cathode. The value of the standard electrode potential is zero, which forms. When a standard hydrogen electrode undergoes. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The cell potential of an electrochemical cell is the difference between its cathode and anode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Standard Hydrogen Electrode Acts As Both Anode And Cathode Why The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. It is used as a reference electrode for determination of standard electrode potential of elements. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Standard Hydrogen Electrode Acts As Both Anode And Cathode Why Examples of using the standard hydrogen electrode to determine reduction potentials are given. When a standard hydrogen electrode undergoes. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. It is used as a reference electrode for determination of standard electrode potential of elements and other. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From diagramlibrarypern.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Standard Hydrogen Electrode Acts As Both Anode And Cathode Why Examples of using the standard hydrogen electrode to determine reduction potentials are given. When a standard hydrogen electrode undergoes. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. It is used as a reference electrode for determination of standard electrode potential of elements and other. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From study.com
Electrolysis of Aqueous Solutions Lesson Standard Hydrogen Electrode Acts As Both Anode And Cathode Why Standard hydrogen electrode can work both as an anode and as a cathode. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. Examples of using the standard hydrogen electrode to determine reduction potentials are given. This is because it acts as a reference for comparison with any other. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.youtube.com
Why Hydrogen electrode acts as both cathode and anode SHE as a Standard Hydrogen Electrode Acts As Both Anode And Cathode Why When a standard hydrogen electrode undergoes. The structure of the standard hydrogen electrode is described. The value of the standard electrode potential is zero, which forms. It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. This is because it acts as a reference for comparison with any other electrode. Examples. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.numerade.com
SOLVED standard hydrogen electrode acts as both anode and cathode Standard Hydrogen Electrode Acts As Both Anode And Cathode Why The cell potential of an electrochemical cell is the difference between its cathode and anode. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k.. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From rohanfersmorrison.blogspot.com
Identify the Conditions for a Standard Electrochemical Cell. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. The cell potential of an electrochemical cell is the difference between its cathode and anode. When a standard hydrogen electrode undergoes. The value of the standard electrode potential is zero, which forms. Graphical representation of the onset potentials of the her/hor. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From slidetodoc.com
Chapter 14 Electrochemistry Oxidation and Reduction reactions redox Standard Hydrogen Electrode Acts As Both Anode And Cathode Why Standard hydrogen electrode can work both as an anode and as a cathode. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. It is used as a reference electrode for determination of standard electrode potential of. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Acts As Both Anode And Cathode Why It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. When a standard hydrogen electrode undergoes. The structure of the standard hydrogen electrode is described. The cell potential of an electrochemical cell is the difference between its cathode and anode. This is because it acts as a reference for comparison with. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Standard Hydrogen Electrode Acts As Both Anode And Cathode Why It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. The cell potential of an electrochemical cell is the difference between its cathode and anode. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. This is because it acts. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.slideserve.com
PPT Topic Electrochemical Cells PowerPoint Presentation, free Standard Hydrogen Electrode Acts As Both Anode And Cathode Why Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The structure of the standard hydrogen electrode is described. It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. The standard hydrogen electrode is often abbreviated to she, and its. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From cider.uoregon.edu
Standard Hydrogen Electrode Demonstration and AACT Simulation CIDER Standard Hydrogen Electrode Acts As Both Anode And Cathode Why It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Standard hydrogen electrode can work both as an anode and as a cathode. When a standard hydrogen. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From mydigitalkemistry.com
Why Standard Hydrogen Electrode act both as a Cathode and Anode Standard Hydrogen Electrode Acts As Both Anode And Cathode Why This is because it acts as a reference for comparison with any other electrode. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode can work both as an anode and as a cathode. When a standard hydrogen electrode undergoes. The cell potential of an electrochemical cell is the difference between its cathode and anode. It is used. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.youtube.com
Identify whether SHE acts as an anode or cathode YouTube Standard Hydrogen Electrode Acts As Both Anode And Cathode Why The value of the standard electrode potential is zero, which forms. This is because it acts as a reference for comparison with any other electrode. When a standard hydrogen electrode undergoes. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. Standard hydrogen electrode can work both as an. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Acts As Both Anode And Cathode Why The value of the standard electrode potential is zero, which forms. It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The structure of the standard hydrogen. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From saylordotorg.github.io
Standard Potentials Standard Hydrogen Electrode Acts As Both Anode And Cathode Why When a standard hydrogen electrode undergoes. The value of the standard electrode potential is zero, which forms. The structure of the standard hydrogen electrode is described. It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From byjus.com
An electrolytic cell is used to convert Standard Hydrogen Electrode Acts As Both Anode And Cathode Why The value of the standard electrode potential is zero, which forms. Standard hydrogen electrode can work both as an anode and as a cathode. This is because it acts as a reference for comparison with any other electrode. When a standard hydrogen electrode undergoes. It is used as a reference electrode for determination of standard electrode potential of elements and. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.batterypowertips.com
What is a cathode? Battery Power Tips Standard Hydrogen Electrode Acts As Both Anode And Cathode Why Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. This is because it acts as a reference for comparison with any other electrode. The value of. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Acts As Both Anode And Cathode Why Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. This is because it acts as a reference for comparison with any other electrode. When a standard hydrogen electrode undergoes. It is used as a reference electrode for determination of standard electrode potential of elements and other half cells.. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.thoughtco.com
How to Define Anode and Cathode Standard Hydrogen Electrode Acts As Both Anode And Cathode Why It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. This is because it acts as a reference for comparison with any other electrode. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. Standard hydrogen electrode can work both. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors Standard Hydrogen Electrode Acts As Both Anode And Cathode Why The value of the standard electrode potential is zero, which forms. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. When a standard hydrogen electrode undergoes. Examples of using the standard hydrogen electrode to determine reduction. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.youtube.com
Cathode and Anode Quick differences and comparisons YouTube Standard Hydrogen Electrode Acts As Both Anode And Cathode Why Standard hydrogen electrode can work both as an anode and as a cathode. The value of the standard electrode potential is zero, which forms. The cell potential of an electrochemical cell is the difference between its cathode and anode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From monomole.com
Standard hydrogen electrode Mono Mole Standard Hydrogen Electrode Acts As Both Anode And Cathode Why The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The structure of the standard hydrogen electrode is described. This is because it acts as. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Acts As Both Anode And Cathode Why The cell potential of an electrochemical cell is the difference between its cathode and anode. When a standard hydrogen electrode undergoes. This is because it acts as a reference for comparison with any other electrode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From byjus.com
What is standard hydrogen electrode? Standard Hydrogen Electrode Acts As Both Anode And Cathode Why When a standard hydrogen electrode undergoes. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. Standard hydrogen electrode can work both as an anode and as a cathode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The cell potential of an electrochemical cell. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.collegesearch.in
Cathode and Anode Definition, Examples, Differences CollegeSearch Standard Hydrogen Electrode Acts As Both Anode And Cathode Why Examples of using the standard hydrogen electrode to determine reduction potentials are given. The value of the standard electrode potential is zero, which forms. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. Standard hydrogen electrode can work both as an anode and as a cathode. When a. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From byjus.com
Why we take notation of SHE as H2/H+ Why not H+/H2 as it can act as Standard Hydrogen Electrode Acts As Both Anode And Cathode Why The cell potential of an electrochemical cell is the difference between its cathode and anode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Standard hydrogen electrode can work both as an anode and as a cathode. The structure of the standard hydrogen electrode is. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Acts As Both Anode And Cathode Why It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. The structure of the standard hydrogen electrode is described. The cell potential of an electrochemical cell is the difference between its cathode and anode. This is because it acts as a reference for comparison with any other electrode. Examples of using. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Standard Hydrogen Electrode Acts As Both Anode And Cathode Why Standard hydrogen electrode can work both as an anode and as a cathode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. This is because it acts as a reference for comparison with any other electrode. The value of the standard electrode potential is zero,. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.youtube.com
Identify anode and cathode Electrochemistry Physical Chemistry Standard Hydrogen Electrode Acts As Both Anode And Cathode Why This is because it acts as a reference for comparison with any other electrode. It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. When a standard hydrogen electrode undergoes. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The value of the standard electrode potential is. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.numerade.com
SOLVED A) Consider the following galvanic cell with a standard Standard Hydrogen Electrode Acts As Both Anode And Cathode Why When a standard hydrogen electrode undergoes. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The cell potential of an electrochemical cell is the. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.slideserve.com
PPT Topic Electrochemical Cells PowerPoint Presentation, free Standard Hydrogen Electrode Acts As Both Anode And Cathode Why The cell potential of an electrochemical cell is the difference between its cathode and anode. When a standard hydrogen electrode undergoes. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Standard hydrogen electrode can work both as an anode and as a cathode. Examples of. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.vedantu.com
Cathode and Anode Definition and Difference Between Anode and Cathode Standard Hydrogen Electrode Acts As Both Anode And Cathode Why This is because it acts as a reference for comparison with any other electrode. Standard hydrogen electrode can work both as an anode and as a cathode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The cell potential of an electrochemical cell is the difference between its cathode and anode. It is used as a. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From www.doubtnut.com
Describe the construction and working of the standard hydrogen electr Standard Hydrogen Electrode Acts As Both Anode And Cathode Why The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. Standard hydrogen electrode can work both as an anode and as a cathode. The value of the standard electrode potential is zero, which forms. It is used as a reference electrode for determination of standard electrode potential of. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.
From gaskatel.de
Principles of the pHvalue measurement with hydrogen electrodes Gaskatel Standard Hydrogen Electrode Acts As Both Anode And Cathode Why Standard hydrogen electrode can work both as an anode and as a cathode. The structure of the standard hydrogen electrode is described. The cell potential of an electrochemical cell is the difference between its cathode and anode. The value of the standard electrode potential is zero, which forms. It is used as a reference electrode for determination of standard electrode. Standard Hydrogen Electrode Acts As Both Anode And Cathode Why.