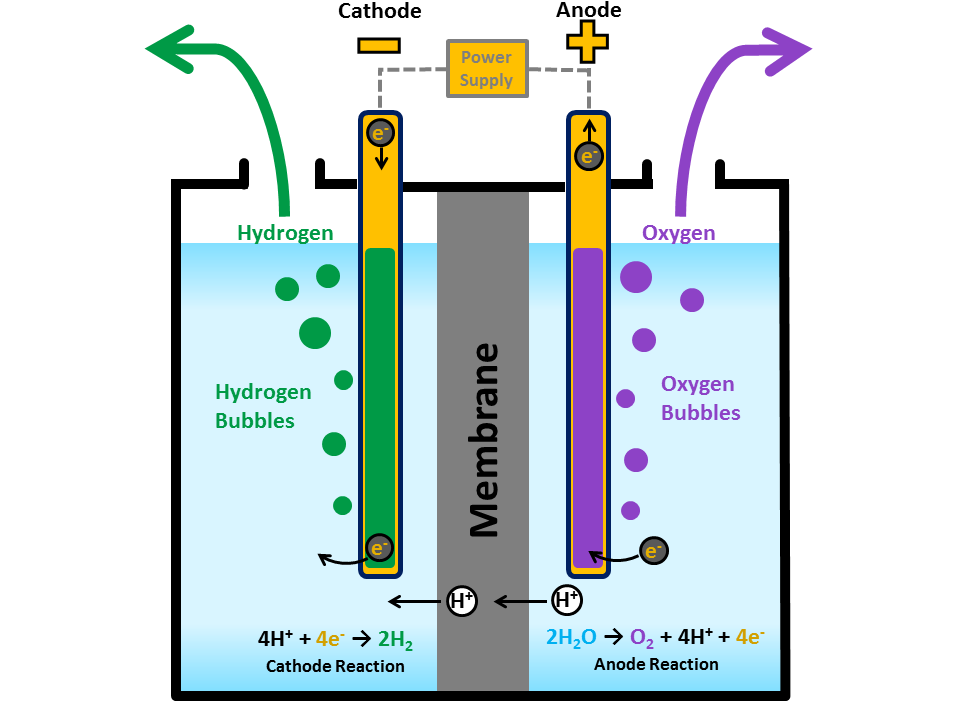
How Does Electrolysis Work Water . Electrolysis of water produces hydrogen and oxygen in a ratio of 2 to 1 respectively. The electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes immersed in water. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. The ions move to the opposite. Water can undergo electrolysis in the presence of an electrolyte like acid or base. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). Electrolysis is the process of using electricity to split water into hydrogen and. The splitting occurs in two partial reactions that take. The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts.
from energy.gov
Water can undergo electrolysis in the presence of an electrolyte like acid or base. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. Electrolysis of water produces hydrogen and oxygen in a ratio of 2 to 1 respectively. Electrolysis is the process of using electricity to split water into hydrogen and. The electrolytic cell consists of a pair of platinum electrodes immersed in water. The splitting occurs in two partial reactions that take. The electrolysis of water produces hydrogen and oxygen gases. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). The ions move to the opposite. The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity.
Hydrogen Production Electrolysis Department of Energy
How Does Electrolysis Work Water The electrolysis of water produces hydrogen and oxygen gases. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. Water can undergo electrolysis in the presence of an electrolyte like acid or base. Electrolysis of water produces hydrogen and oxygen in a ratio of 2 to 1 respectively. The ions move to the opposite. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). The splitting occurs in two partial reactions that take. The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. Electrolysis is the process of using electricity to split water into hydrogen and. The electrolysis of water produces hydrogen and oxygen gases.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID2216212 How Does Electrolysis Work Water Electrolysis is the process of using electricity to split water into hydrogen and. The electrolysis of water produces hydrogen and oxygen gases. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Water can undergo electrolysis in the presence of. How Does Electrolysis Work Water.
From www.walkaline.biz
How Water Electrolysis and Ionizer Works WALKALINE How Does Electrolysis Work Water Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The electrolysis of water produces hydrogen and oxygen gases. The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ).. How Does Electrolysis Work Water.
From scienceinfo.com
Electrolysis of Water Definition, Principle, and Applications How Does Electrolysis Work Water Water can undergo electrolysis in the presence of an electrolyte like acid or base. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. Electrolysis is the process of using electricity to split water into hydrogen and. The ions move to the opposite. Electrolysis of water produces hydrogen and oxygen in a ratio. How Does Electrolysis Work Water.
From en.ppt-online.org
Electrolysis online presentation How Does Electrolysis Work Water Water can undergo electrolysis in the presence of an electrolyte like acid or base. Electrolysis of water produces hydrogen and oxygen in a ratio of 2 to 1 respectively. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. The ions move to the opposite. The electrolysis of water produces hydrogen and oxygen. How Does Electrolysis Work Water.
From www.scienceabc.com
How Does The ISS Get Air (Breathable Oxygen)? How Does Electrolysis Work Water Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The splitting occurs in two partial reactions that take. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. Electrolysis is the process of using electricity to split water into hydrogen and. The basic principle of. How Does Electrolysis Work Water.
From chemistry.stackexchange.com
Why does adding an acid speed up electrolysis of water? Chemistry Stack Exchange How Does Electrolysis Work Water The electrolytic cell consists of a pair of platinum electrodes immersed in water. The splitting occurs in two partial reactions that take. The electrolysis of water produces hydrogen and oxygen gases. Electrolysis is the process of using electricity to split water into hydrogen and. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ).. How Does Electrolysis Work Water.
From www.wikihow.com
How to Electrolyse Water 12 Steps (with Pictures) wikiHow How Does Electrolysis Work Water The splitting occurs in two partial reactions that take. The ions move to the opposite. The electrolysis of water produces hydrogen and oxygen gases. Water can undergo electrolysis in the presence of an electrolyte like acid or base. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. Electrolysis is the process of. How Does Electrolysis Work Water.
From morrisclassicalacademy.blogspot.com
Morris Classical Academy Electrolysis of water How Does Electrolysis Work Water The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). Electrolysis is the process of using electricity to split water into hydrogen and. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Water can undergo electrolysis in the presence of an electrolyte like acid or base. The electrolysis of water. How Does Electrolysis Work Water.
From www.freepik.com
Free Vector Electrolysis diagram experiment for education How Does Electrolysis Work Water Electrolysis is the process of using electricity to split water into hydrogen and. The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. The ions move to the opposite. Water can undergo electrolysis in the presence of an electrolyte like acid or base. The electrolytic cell consists of a pair of platinum. How Does Electrolysis Work Water.
From www.pinterest.com
Electrolysis Definition, Process, Equations, Examples, and Applications in 2022 Equations How Does Electrolysis Work Water The ions move to the opposite. The splitting occurs in two partial reactions that take. Electrolysis is the process of using electricity to split water into hydrogen and. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Electrolysis of. How Does Electrolysis Work Water.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace How Does Electrolysis Work Water Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). The electrolytic cell consists of a pair of platinum electrodes. How Does Electrolysis Work Water.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk How Does Electrolysis Work Water Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. Water can undergo electrolysis in the presence of an electrolyte like acid or base. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). The basic principle of electrolysis is to split water into oxygen and hydrogen. How Does Electrolysis Work Water.
From www.dreamstime.com
Electrolysis of Water Splitting H2O Molecules Stock Illustration Illustration of vibrant How Does Electrolysis Work Water The ions move to the opposite. The electrolysis of water produces hydrogen and oxygen gases. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). Electrolysis is the process of using electricity to split water into hydrogen and. The splitting occurs in two partial reactions that take. Water can undergo electrolysis in the presence. How Does Electrolysis Work Water.
From geniebook.com
Electrolysis Secondary 4 Chemistry Geniebook How Does Electrolysis Work Water Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The splitting occurs in two partial reactions that take. Electrolysis of water produces hydrogen and oxygen in a ratio of 2 to 1 respectively. The electrolytic cell consists of a pair of platinum electrodes immersed in water. The presence of acid improves the. How Does Electrolysis Work Water.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts How Does Electrolysis Work Water Electrolysis is the process of using electricity to split water into hydrogen and. The splitting occurs in two partial reactions that take. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. The basic principle of. How Does Electrolysis Work Water.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk How Does Electrolysis Work Water The splitting occurs in two partial reactions that take. The electrolytic cell consists of a pair of platinum electrodes immersed in water. The electrolysis of water produces hydrogen and oxygen gases. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The basic principle of electrolysis is to split water into oxygen and. How Does Electrolysis Work Water.
From www.youtube.com
The Electrolysis Of Water (GCSE Chemistry) YouTube How Does Electrolysis Work Water 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The splitting occurs in two partial reactions that take. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). The. How Does Electrolysis Work Water.
From byjus.com
Water Electrolysis Principle of Water Electrolysis, Important Factors, Electrolytes How Does Electrolysis Work Water The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. Electrolysis is the process of using electricity to split water into hydrogen and. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. 2 h 2 o(l) → 2 h 2 (g) + o 2. How Does Electrolysis Work Water.
From study.com
Electrolysis of Aqueous Solutions Lesson How Does Electrolysis Work Water The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The electrolytic cell consists of a pair of platinum electrodes immersed in water. The splitting occurs in two partial reactions that take. The basic principle of electrolysis. How Does Electrolysis Work Water.
From www.chemicals.co.uk
What is a Chemical Change? The Chemistry Blog How Does Electrolysis Work Water The ions move to the opposite. The electrolysis of water produces hydrogen and oxygen gases. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The presence of acid improves the electrical conductivity by increasing the. How Does Electrolysis Work Water.
From www.chemistrylearner.com
Analytical Chemistry Chemistry Learner How Does Electrolysis Work Water The splitting occurs in two partial reactions that take. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. The electrolysis of water produces hydrogen and oxygen gases. Electrolysis of water produces hydrogen and oxygen. How Does Electrolysis Work Water.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps How Does Electrolysis Work Water Electrolysis is the process of using electricity to split water into hydrogen and. The splitting occurs in two partial reactions that take. The ions move to the opposite. The electrolysis of water produces hydrogen and oxygen gases. Electrolysis of water produces hydrogen and oxygen in a ratio of 2 to 1 respectively. The electrolytic cell consists of a pair of. How Does Electrolysis Work Water.
From www.pinterest.com
Water electrolysis process scientific chemistry diagram VectorMine Water electrolysis How Does Electrolysis Work Water The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. Electrolysis is the process of using electricity to split water into hydrogen and. The electrolysis of water produces hydrogen and oxygen gases. The splitting occurs in two partial reactions that take. Water can undergo electrolysis in the presence of an electrolyte like. How Does Electrolysis Work Water.
From www.oceangeothermal.org
Hydrogen Through Electrolysis Ocean Geothermal Energy Foundation How Does Electrolysis Work Water The electrolysis of water produces hydrogen and oxygen gases. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. Electrolysis is the process of using electricity to split water into hydrogen and. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The electrolytic cell consists. How Does Electrolysis Work Water.
From www.youtube.com
WATER ELECTROLYSIS DEMONSTRATION WITH EXPLANATION CHEMISTRY GRADE 812 YouTube How Does Electrolysis Work Water The electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Electrolysis of water produces hydrogen and oxygen in a ratio of 2 to 1 respectively. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The presence of acid improves the. How Does Electrolysis Work Water.
From www.youtube.com
Electrolysis of Water Electrochemistry YouTube How Does Electrolysis Work Water The electrolytic cell consists of a pair of platinum electrodes immersed in water. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). Water can undergo electrolysis in the presence of an electrolyte like acid or base.. How Does Electrolysis Work Water.
From pricklynomore.com
The science of electrolysis — Prickly Pear Electrolysis & Skincare How Does Electrolysis Work Water The electrolytic cell consists of a pair of platinum electrodes immersed in water. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The ions move to the opposite. Electrolysis of water produces hydrogen and oxygen in a ratio of 2 to 1 respectively. Electrolysis is the process of using electricity to split. How Does Electrolysis Work Water.
From enagic-asia.com
The electrolysis process Enagic Kangen Water How Does Electrolysis Work Water Water can undergo electrolysis in the presence of an electrolyte like acid or base. The splitting occurs in two partial reactions that take. The ions move to the opposite. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. Electrolysis is the process of using electricity to split water into hydrogen and. 2. How Does Electrolysis Work Water.
From energy.gov
Hydrogen Production Electrolysis Department of Energy How Does Electrolysis Work Water The splitting occurs in two partial reactions that take. Water can undergo electrolysis in the presence of an electrolyte like acid or base. The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. The electrolysis of water produces hydrogen and oxygen gases. Electrolysis is a technique used by scientists to separate a. How Does Electrolysis Work Water.
From mungfali.com
Electrolysis Process Diagram How Does Electrolysis Work Water 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). The electrolytic cell consists of a pair of platinum electrodes immersed in water. The electrolysis of water produces hydrogen and oxygen gases. Electrolysis is a technique used. How Does Electrolysis Work Water.
From www.researchgate.net
The fundamental of water electrolysis process. Download Scientific Diagram How Does Electrolysis Work Water Electrolysis of water produces hydrogen and oxygen in a ratio of 2 to 1 respectively. The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. The ions move to the opposite. The splitting occurs in two partial reactions that take. The presence of acid improves the electrical conductivity by increasing the hydrogen. How Does Electrolysis Work Water.
From theedge.com.hk
waterelectrolysis The Edge How Does Electrolysis Work Water The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. Electrolysis is the process of using electricity to split. How Does Electrolysis Work Water.
From www.academia.edu
(PDF) Electrolysis Process of Water and How It Works satu hitotsu Academia.edu How Does Electrolysis Work Water 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (h + ). The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. Water can undergo electrolysis in the presence of an. How Does Electrolysis Work Water.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes PtX Hub How Does Electrolysis Work Water 2 h 2 o(l) → 2 h 2 (g) + o 2 (g) e° = +1.229 v. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. The electrolytic cell consists of a pair of. How Does Electrolysis Work Water.
From www.alamy.com
electrolysis cell of water to produce hydrogen and oxygen Stock Photo Alamy How Does Electrolysis Work Water The electrolysis of water produces hydrogen and oxygen gases. Electrolysis of water produces hydrogen and oxygen in a ratio of 2 to 1 respectively. Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. The ions move to the opposite. The basic principle of electrolysis is to split water into oxygen and hydrogen. How Does Electrolysis Work Water.