Chlorine And Bromine Ionic Or Covalent . for example, consider binary ionic compounds of iron and chlorine. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. for example, consider binary ionic compounds of iron and chlorine. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. aluminum and chlorine react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of. Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic and. Iron typically exhibits a charge of either 2+ or.
from www.numerade.com
Iron typically exhibits a charge of either 2+ or. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic and. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. for example, consider binary ionic compounds of iron and chlorine. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. aluminum and chlorine react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of. for example, consider binary ionic compounds of iron and chlorine.
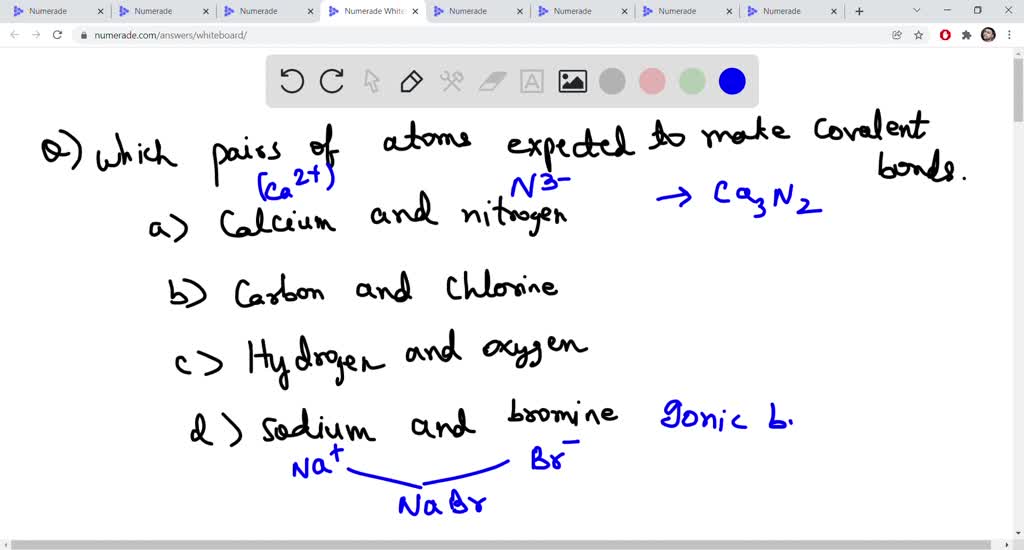
SOLVED Which of the pairs of atoms below would be expected to make covalent bonds? Select all
Chlorine And Bromine Ionic Or Covalent Iron typically exhibits a charge of either 2+ or. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Iron typically exhibits a charge of either 2+ or. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Predict which forms an anion, which forms a cation, and the charges of. for example, consider binary ionic compounds of iron and chlorine. for example, consider binary ionic compounds of iron and chlorine. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic and. aluminum and chlorine react to form an ionic compound.
From slideplayer.com
Chapter 6 Chemical Bonding. ppt download Chlorine And Bromine Ionic Or Covalent for example, consider binary ionic compounds of iron and chlorine. aluminum and chlorine react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Iron typically exhibits a charge of. Chlorine And Bromine Ionic Or Covalent.
From www.slideserve.com
PPT iGCSE chemistry Section 2 lesson 2 PowerPoint Presentation, free download ID9583872 Chlorine And Bromine Ionic Or Covalent for example, consider binary ionic compounds of iron and chlorine. for example, consider binary ionic compounds of iron and chlorine. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2. Chlorine And Bromine Ionic Or Covalent.
From www.slideserve.com
PPT Chapter Twenty PowerPoint Presentation, free download ID6301153 Chlorine And Bromine Ionic Or Covalent for example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. note, members of the same family tend to form similar compounds, so bromine and iodine form similar. Chlorine And Bromine Ionic Or Covalent.
From www.slideserve.com
PPT PART I PowerPoint Presentation, free download ID2826467 Chlorine And Bromine Ionic Or Covalent Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic and. aluminum and chlorine react to form an ionic compound. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. covalent bonds are the attractive forces between the positively. Chlorine And Bromine Ionic Or Covalent.
From questions-in.kunduz.com
4.34 Identify the bonds formed between t... Chemistry Chlorine And Bromine Ionic Or Covalent for example, consider binary ionic compounds of iron and chlorine. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Predict which forms an anion, which forms a cation, and the charges of. aluminum and chlorine react to form an ionic compound. Iron typically exhibits a. Chlorine And Bromine Ionic Or Covalent.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as diatomic molecules. Oxygen Chlorine And Bromine Ionic Or Covalent note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. for example, consider binary ionic compounds of iron and chlorine. aluminum and chlorine react to form an ionic compound. Iron typically exhibits a charge of either 2+ or. Iron typically exhibits a charge of either 2+. Chlorine And Bromine Ionic Or Covalent.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties Chlorine And Bromine Ionic Or Covalent Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic and. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet.. Chlorine And Bromine Ionic Or Covalent.
From edu.rsc.org
Reactions of chlorine, bromine and iodine with aluminium Experiment RSC Education Chlorine And Bromine Ionic Or Covalent note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. for example, consider binary ionic compounds of iron and chlorine. covalent bonds are the attractive forces. Chlorine And Bromine Ionic Or Covalent.
From www.alamy.com
Fluorine, Bromine and Chlorine Molecular Model of Atom. Vector illustration Stock Vector Image Chlorine And Bromine Ionic Or Covalent note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one. Chlorine And Bromine Ionic Or Covalent.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points, Definitions, Examples Chlorine And Bromine Ionic Or Covalent for example, consider binary ionic compounds of iron and chlorine. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Predict which forms an anion, which forms a cation, and the charges of. aluminum and chlorine react to form an ionic compound. when forming ions,. Chlorine And Bromine Ionic Or Covalent.
From www.numerade.com
SOLVED Which of the pairs of atoms below would be expected to make covalent bonds? Select all Chlorine And Bromine Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. for example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or. aluminum and chlorine react to form. Chlorine And Bromine Ionic Or Covalent.
From printabletiroes.z22.web.core.windows.net
Describe The Different Types Of Bonds Chlorine And Bromine Ionic Or Covalent aluminum and chlorine react to form an ionic compound. Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic and. for example, consider binary ionic compounds of iron and chlorine. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine. Chlorine And Bromine Ionic Or Covalent.
From chemistrylearnwithsangam.blogspot.com
chemistry knowledge Comparison between Covalent and Ionic Bond Chlorine And Bromine Ionic Or Covalent for example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or. aluminum and chlorine react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of. Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic. Chlorine And Bromine Ionic Or Covalent.
From diagramdataperianths.z21.web.core.windows.net
Diagram Ionic Bond Chlorine And Bromine Ionic Or Covalent for example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. note, members of the same family tend to form similar compounds, so bromine and iodine form similar. Chlorine And Bromine Ionic Or Covalent.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Chlorine And Bromine Ionic Or Covalent covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. aluminum and chlorine react to form an ionic compound. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. for example, consider binary ionic. Chlorine And Bromine Ionic Or Covalent.
From slideplayer.com
The Chemistry of Life Chapter 2 ppt download Chlorine And Bromine Ionic Or Covalent for example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic and. for example, consider binary ionic compounds of iron and chlorine. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet.. Chlorine And Bromine Ionic Or Covalent.
From guidelistitinfinitary.z13.web.core.windows.net
Lewis Diagram Ion Chlorine And Bromine Ionic Or Covalent note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2. Chlorine And Bromine Ionic Or Covalent.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Chlorine And Bromine Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. note, members of the. Chlorine And Bromine Ionic Or Covalent.
From www.poolsuppliessuperstore.com
Bromine vs. Chlorine Everything You Need to Know Pool Supplies Superstore Chlorine And Bromine Ionic Or Covalent for example, consider binary ionic compounds of iron and chlorine. Predict which forms an anion, which forms a cation, and the charges of. Iron typically exhibits a charge of either 2+ or. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. note, members of the. Chlorine And Bromine Ionic Or Covalent.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b)... Download Scientific Diagram Chlorine And Bromine Ionic Or Covalent when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Iron typically exhibits a charge of either 2+ or. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. for example, consider binary ionic compounds of iron. Chlorine And Bromine Ionic Or Covalent.
From www2.chemistry.msu.edu
Electron Configurations & The Periodic Table Chlorine And Bromine Ionic Or Covalent Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic and. for example, consider binary ionic compounds of iron and chlorine. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. covalent bonds are the attractive forces between the. Chlorine And Bromine Ionic Or Covalent.
From dokumen.tips
(PDF) Single Covalent Bonds · Draw electron dot structures for each molecule. Chlorine gas Chlorine And Bromine Ionic Or Covalent when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic and. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of.. Chlorine And Bromine Ionic Or Covalent.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Chlorine And Bromine Ionic Or Covalent Iron typically exhibits a charge of either 2+ or. for example, consider binary ionic compounds of iron and chlorine. aluminum and chlorine react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of. note, members of the same family tend to form similar compounds, so bromine and iodine form. Chlorine And Bromine Ionic Or Covalent.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen, hydrogen, nitrogen, fluorine Chlorine And Bromine Ionic Or Covalent when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. for example, consider binary ionic compounds of iron and chlorine. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Predict which forms an anion, which forms. Chlorine And Bromine Ionic Or Covalent.
From feedwater.co.uk
The Feedwater Knowledge base Important water treatment information and news Chlorine And Bromine Ionic Or Covalent when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. for example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more. Chlorine And Bromine Ionic Or Covalent.
From www.buzzle.com
Bromine Vs. Chlorine Chlorine And Bromine Ionic Or Covalent Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic and. for example, consider binary ionic compounds of iron and chlorine. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. for example, consider binary ionic compounds of iron. Chlorine And Bromine Ionic Or Covalent.
From pediaa.com
Difference Between Bromine and Chlorine Chlorine And Bromine Ionic Or Covalent when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. aluminum and chlorine react to form an ionic compound. Iron typically exhibits a charge of either 2+ or. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs. Chlorine And Bromine Ionic Or Covalent.
From www.revisechemistry.uk
Bonding and Properties of materials OCR Gateway C2 revisechemistry.uk Chlorine And Bromine Ionic Or Covalent aluminum and chlorine react to form an ionic compound. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. for example, consider binary ionic. Chlorine And Bromine Ionic Or Covalent.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Resources Chlorine And Bromine Ionic Or Covalent Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic and. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. aluminum and chlorine react to form an ionic compound. when forming ions, elements typically gain or lose the. Chlorine And Bromine Ionic Or Covalent.
From wisc.pb.unizin.org
M8Q3 Resonance Structures and Formal Charge Chem 103/104 Resource Book Chlorine And Bromine Ionic Or Covalent when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. for example, consider binary ionic compounds of iron and chlorine. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Iron typically exhibits a charge of either. Chlorine And Bromine Ionic Or Covalent.
From www.numerade.com
SOLVED Classify each of the following bonds as ionic, polar covalent, or nonpolar covalent Chlorine And Bromine Ionic Or Covalent Iron typically exhibits a charge of either 2+ or 3+ (see figure 2 in chapter 4.2 ionic and. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. aluminum and chlorine react to form an ionic compound. for example, consider binary ionic compounds of iron and. Chlorine And Bromine Ionic Or Covalent.
From exofhdgch.blob.core.windows.net
Bromine Ionic Or Covalent at Donna McMillian blog Chlorine And Bromine Ionic Or Covalent aluminum and chlorine react to form an ionic compound. Iron typically exhibits a charge of either 2+ or. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs. Chlorine And Bromine Ionic Or Covalent.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Chlorine And Bromine Ionic Or Covalent note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. for example, consider binary ionic compounds of iron and chlorine. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Predict which forms an anion,. Chlorine And Bromine Ionic Or Covalent.
From www.differencebetween.com
Difference Between Bromine and Chlorine Compare the Difference Between Similar Terms Chlorine And Bromine Ionic Or Covalent covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. for example, consider binary ionic compounds of iron and chlorine. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. aluminum and chlorine react. Chlorine And Bromine Ionic Or Covalent.
From www.numerade.com
SOLVED Using the electronegativity chart, identify the bond type between chlorine (Cl) and Chlorine And Bromine Ionic Or Covalent for example, consider binary ionic compounds of iron and chlorine. Predict which forms an anion, which forms a cation, and the charges of. Iron typically exhibits a charge of either 2+ or. for example, consider binary ionic compounds of iron and chlorine. when forming ions, elements typically gain or lose the minimum number of electrons necessary to. Chlorine And Bromine Ionic Or Covalent.