Aluminum Hydroxide And Hcl Balanced Equation . The stoichiometric proportion of hydroxide particles (oh− o h −) to aluminum particles (al3+ a l 3 +). First, we set all coefficients to 1: In this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. Find out what type of reaction occured. The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq) + al(oh) 3 (s) ==> alcl 3 (aq) + 3h2o(l). Let's balance this equation using the inspection method. Balance any equation or reaction using this chemical equation balancer! Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each.
from www.numerade.com
Balance any equation or reaction using this chemical equation balancer! In this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. Let's balance this equation using the inspection method. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq) + al(oh) 3 (s) ==> alcl 3 (aq) + 3h2o(l). First, we set all coefficients to 1: 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. The stoichiometric proportion of hydroxide particles (oh− o h −) to aluminum particles (al3+ a l 3 +). Find out what type of reaction occured. The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride.
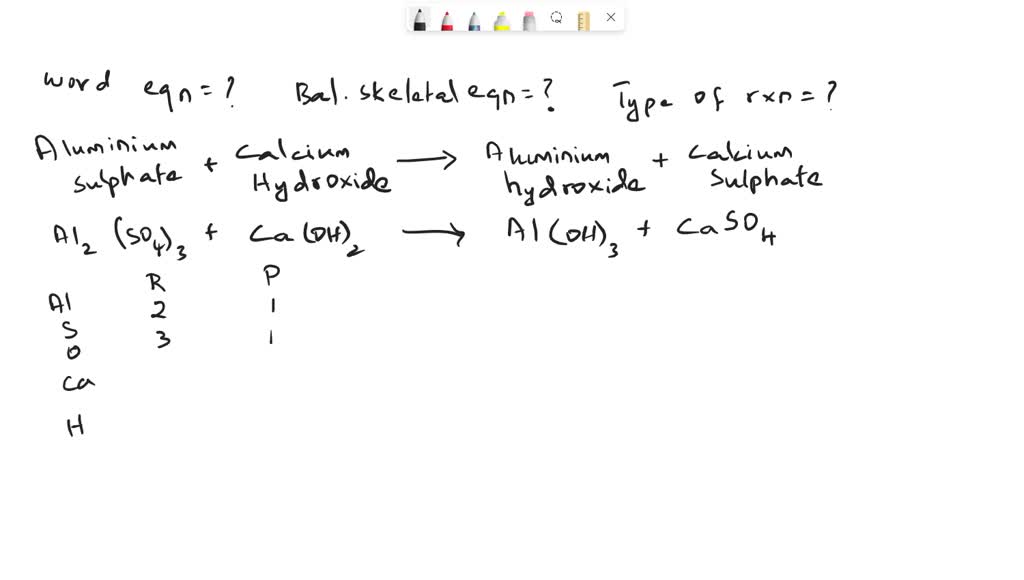
SOLVED Aluminum sulfate solution and calcium hydroxide solution
Aluminum Hydroxide And Hcl Balanced Equation Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. First, we set all coefficients to 1: Find out what type of reaction occured. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. Let's balance this equation using the inspection method. 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. Balance any equation or reaction using this chemical equation balancer! The stoichiometric proportion of hydroxide particles (oh− o h −) to aluminum particles (al3+ a l 3 +). Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq) + al(oh) 3 (s) ==> alcl 3 (aq) + 3h2o(l). In this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride.
From www.numerade.com
SOLVED From the following balanced equation, the mass of aluminum Aluminum Hydroxide And Hcl Balanced Equation Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. The stoichiometric proportion of hydroxide particles (oh− o h −) to aluminum particles (al3+ a l 3 +).. Aluminum Hydroxide And Hcl Balanced Equation.
From www.youtube.com
Balancing Chemical Equations. Part 1 Aluminium and hydrochloric acid Aluminum Hydroxide And Hcl Balanced Equation The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. Hydrochloric acid. Aluminum Hydroxide And Hcl Balanced Equation.
From www.numerade.com
SOLVED Aluminum hydroxide can be produced by the following reaction in Aluminum Hydroxide And Hcl Balanced Equation Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. First, we set all coefficients to 1: In this video we'll balance the equation al (oh)3 + hcl =. Aluminum Hydroxide And Hcl Balanced Equation.
From www.numerade.com
SOLVED Aluminum sulfate solution and calcium hydroxide solution Aluminum Hydroxide And Hcl Balanced Equation Let's balance this equation using the inspection method. Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq) + al(oh) 3 (s) ==> alcl 3 (aq) + 3h2o(l). Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. In this video we'll balance the. Aluminum Hydroxide And Hcl Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Al(OH)3 + HBr = AlBr3 + H2O Aluminum Hydroxide And Hcl Balanced Equation Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq) + al(oh) 3 (s) ==> alcl 3 (aq) + 3h2o(l). 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. Balance any equation or reaction using this chemical equation balancer! First, we set all coefficients to 1: The stoichiometric proportion of hydroxide. Aluminum Hydroxide And Hcl Balanced Equation.
From www.youtube.com
Equation for Al(OH)3 + H2O (Aluminum Hydroxide + Water) YouTube Aluminum Hydroxide And Hcl Balanced Equation The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. In this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. Al + hcl = alcl3 + h2 is a single displacement (substitution). Aluminum Hydroxide And Hcl Balanced Equation.
From indianewsmack.blogspot.com
Hydrochloric Acid and Sodium Hydroxide Balanced Equation With States Aluminum Hydroxide And Hcl Balanced Equation First, we set all coefficients to 1: 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. The balanced equation shows the reaction between aluminium hydroxide and hydrochloric. Aluminum Hydroxide And Hcl Balanced Equation.
From shapeguidance1.gitlab.io
Glory Hcl And Sodium Hydroxide Balanced Equation Physics Book Class 12 Aluminum Hydroxide And Hcl Balanced Equation Find out what type of reaction occured. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. First, we set all coefficients to 1: The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. Let's balance this equation using. Aluminum Hydroxide And Hcl Balanced Equation.
From asideload7.gitlab.io
Ideal Balanced Chemical Equation Hcl And Naoh Equations Worksheet With Aluminum Hydroxide And Hcl Balanced Equation Find out what type of reaction occured. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. First, we set all coefficients to 1: Let's balance this equation using the inspection method. In this video we'll balance the equation al (oh)3 + hcl = alcl3. Aluminum Hydroxide And Hcl Balanced Equation.
From www.numerade.com
SOLVED 'Hydrochloric Acid Sodium Hydroxide no rra(tiun lirav Aluminum Hydroxide And Hcl Balanced Equation Balance any equation or reaction using this chemical equation balancer! Let's balance this equation using the inspection method. The stoichiometric proportion of hydroxide particles (oh− o h −) to aluminum particles (al3+ a l 3 +). First, we set all coefficients to 1: Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of. Aluminum Hydroxide And Hcl Balanced Equation.
From www.youtube.com
FeCl3+H2O=Fe(OH)3+HCl. balance the chemical equation mydocumentary838 Aluminum Hydroxide And Hcl Balanced Equation 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. Let's balance this equation using the inspection method. In this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. Al + hcl =. Aluminum Hydroxide And Hcl Balanced Equation.
From www.numerade.com
SOLVED a) Write the balanced chemical reaction for the following (2 Aluminum Hydroxide And Hcl Balanced Equation 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. Find out. Aluminum Hydroxide And Hcl Balanced Equation.
From www.numerade.com
SOLVED(d) Aluminum hydroxide is an over the counterantacid that reacts Aluminum Hydroxide And Hcl Balanced Equation Find out what type of reaction occured. First, we set all coefficients to 1: Balance any equation or reaction using this chemical equation balancer! In this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. Aluminum Hydroxide And Hcl Balanced Equation.
From www.numerade.com
SOLVEDAluminum hydroxide reacts with phosphoric acid to give AlPO4 Aluminum Hydroxide And Hcl Balanced Equation Balance any equation or reaction using this chemical equation balancer! 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. The stoichiometric proportion of hydroxide particles (oh− o. Aluminum Hydroxide And Hcl Balanced Equation.
From www.meritnation.com
What is the coefficient of sodium hydroxide in a balanced chemical Aluminum Hydroxide And Hcl Balanced Equation In this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. Find out what type of reaction occured. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq) + al(oh). Aluminum Hydroxide And Hcl Balanced Equation.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Aluminum Hydroxide And Hcl Balanced Equation Find out what type of reaction occured. 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. In this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. Al + hcl = alcl3. Aluminum Hydroxide And Hcl Balanced Equation.
From www.solutionspile.com
[Solved] Write the balanced chemical equation for the reac Aluminum Hydroxide And Hcl Balanced Equation 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. First, we set all coefficients to 1: In this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. The stoichiometric proportion of hydroxide particles (oh− o h −) to aluminum particles (al3+ a l 3 +). Find out what. Aluminum Hydroxide And Hcl Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction between Aluminum Hydroxide And Hcl Balanced Equation Find out what type of reaction occured. First, we set all coefficients to 1: Balance any equation or reaction using this chemical equation balancer! Let's balance this equation using the inspection method. Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq) + al(oh) 3 (s) ==> alcl 3 (aq) + 3h2o(l). The balanced equation shows the reaction between. Aluminum Hydroxide And Hcl Balanced Equation.
From byjus.com
The balanced net ionic equation for the reaction of aluminium sulphate Aluminum Hydroxide And Hcl Balanced Equation Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq) + al(oh) 3 (s) ==> alcl 3 (aq) + 3h2o(l). Find out what type of reaction occured. The stoichiometric proportion of hydroxide particles (oh− o h −) to aluminum particles (al3+ a l 3 +). 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl. Aluminum Hydroxide And Hcl Balanced Equation.
From www.youtube.com
How to Balance Al(OH)3 + HCl = AlCl3 + H2O Aluminum hydroxide Aluminum Hydroxide And Hcl Balanced Equation The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. First, we set all coefficients to 1: Let's balance this equation using the inspection method. Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq) + al(oh) 3 (s) ==> alcl 3 (aq) + 3h2o(l). The stoichiometric proportion of hydroxide particles (oh− o. Aluminum Hydroxide And Hcl Balanced Equation.
From www.youtube.com
HNO3+Al(OH)3=Al(NO3)3+H2O Balanced EquationNitric acid+Aluminum Aluminum Hydroxide And Hcl Balanced Equation Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. Balance any equation or reaction using this chemical equation balancer! 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. The stoichiometric proportion of hydroxide particles (oh− o. Aluminum Hydroxide And Hcl Balanced Equation.
From www.youtube.com
How to Write the Formula for Aluminum hydroxide YouTube Aluminum Hydroxide And Hcl Balanced Equation Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. Find out what type of reaction occured. Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq). Aluminum Hydroxide And Hcl Balanced Equation.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between calcium Aluminum Hydroxide And Hcl Balanced Equation First, we set all coefficients to 1: The stoichiometric proportion of hydroxide particles (oh− o h −) to aluminum particles (al3+ a l 3 +). Let's balance this equation using the inspection method. Find out what type of reaction occured. 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. Al + hcl. Aluminum Hydroxide And Hcl Balanced Equation.
From www.youtube.com
Type of Reaction for Al(OH)3 = Al2O3 + H2O (at high temps) YouTube Aluminum Hydroxide And Hcl Balanced Equation The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. The stoichiometric. Aluminum Hydroxide And Hcl Balanced Equation.
From www.youtube.com
How to Balance NaOH + HCl = NaCl + H2O (Sodium Hydroxide Plus Aluminum Hydroxide And Hcl Balanced Equation 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. Let's balance this equation using the inspection method. The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. Balance any equation or reaction using this chemical equation balancer! The stoichiometric proportion of hydroxide particles (oh− o h. Aluminum Hydroxide And Hcl Balanced Equation.
From www.youtube.com
MgSO4+NaOH=Mg(OH)2+Na2SO4. balance the chemical equation Aluminum Hydroxide And Hcl Balanced Equation First, we set all coefficients to 1: Balance any equation or reaction using this chemical equation balancer! Let's balance this equation using the inspection method. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. In this video we'll balance the equation al (oh)3 +. Aluminum Hydroxide And Hcl Balanced Equation.
From www.numerade.com
SOLVEDWrite the balanced equation for the reaction of slaked lime Aluminum Hydroxide And Hcl Balanced Equation Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. First, we. Aluminum Hydroxide And Hcl Balanced Equation.
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free Aluminum Hydroxide And Hcl Balanced Equation First, we set all coefficients to 1: Let's balance this equation using the inspection method. Find out what type of reaction occured. Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq) + al(oh) 3 (s) ==> alcl 3 (aq) + 3h2o(l). The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. In. Aluminum Hydroxide And Hcl Balanced Equation.
From www.numerade.com
SOLVED Be sure to answer all parts. Some liquid antacids contain Aluminum Hydroxide And Hcl Balanced Equation The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. The stoichiometric proportion of hydroxide particles (oh− o h −) to aluminum particles (al3+ a l 3 +). 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. Let's balance this equation using the inspection method. Al. Aluminum Hydroxide And Hcl Balanced Equation.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Aluminum Hydroxide And Hcl Balanced Equation Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. The balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium chloride. The stoichiometric proportion of hydroxide particles (oh− o h −) to aluminum particles (al3+ a l 3 +). In. Aluminum Hydroxide And Hcl Balanced Equation.
From www.slideserve.com
PPT Mg(OH) 2 + 2HCl → 2H 2 O + MgCl 2 PowerPoint Presentation, free Aluminum Hydroxide And Hcl Balanced Equation Balance any equation or reaction using this chemical equation balancer! Let's balance this equation using the inspection method. The stoichiometric proportion of hydroxide particles (oh− o h −) to aluminum particles (al3+ a l 3 +). Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of. Aluminum Hydroxide And Hcl Balanced Equation.
From www.numerade.com
SOLVEDand net ionic equation for the following eactions Write Aluminum Hydroxide And Hcl Balanced Equation 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. Find out what type of reaction occured. Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq). Aluminum Hydroxide And Hcl Balanced Equation.
From www.chegg.com
Solved .(18 points) Aluminum hydroxide reacts with Aluminum Hydroxide And Hcl Balanced Equation Balance any equation or reaction using this chemical equation balancer! In this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. First, we set all coefficients to 1: Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq) + al(oh) 3 (s) ==> alcl 3 (aq) + 3h2o(l). Al + hcl = alcl3 + h2. Aluminum Hydroxide And Hcl Balanced Equation.
From www.slideserve.com
PPT First write a balanced equation. PowerPoint Presentation, free Aluminum Hydroxide And Hcl Balanced Equation The stoichiometric proportion of hydroxide particles (oh− o h −) to aluminum particles (al3+ a l 3 +). 1 hcl + 1 al(oh) 3 = 1 h 2 o + 1 alcl 3 for each. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous.. Aluminum Hydroxide And Hcl Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced molecular equation and a net ionic equation for Aluminum Hydroxide And Hcl Balanced Equation Let's balance this equation using the inspection method. Hydrochloric acid = hcl(aq) aluminum hydroxide = al(oh) 3 (s) 3hcl(aq) + al(oh) 3 (s) ==> alcl 3 (aq) + 3h2o(l). First, we set all coefficients to 1: Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of. Aluminum Hydroxide And Hcl Balanced Equation.