What Happens When Gold Reacts With Hydrochloric Acid . Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1. This page shows how the position of a metal in the reactivity series affects its reactions with common dilute acids such as hydrochloric acid. It is produced when sodium reacts with hydrochloric acid. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. Reaction of gold with acids. But when \muriatic acid is For example, zinc reacts with hydro chloric acid to produce zinc chloride. When metals react with acids, a salt of the metal and hydrogen gas are formed. When gold is subjected to treatment with muriatic acid alone, nothing happens. Gold does not dissolve in nitric acid, hno 3 [4]. Reactions of metals with acid.
from www.numerade.com
Reactions of metals with acid. For example, zinc reacts with hydro chloric acid to produce zinc chloride. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen. It is produced when sodium reacts with hydrochloric acid. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. When gold is subjected to treatment with muriatic acid alone, nothing happens. Reaction of gold with acids. But when \muriatic acid is When metals react with acids, a salt of the metal and hydrogen gas are formed. Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1.
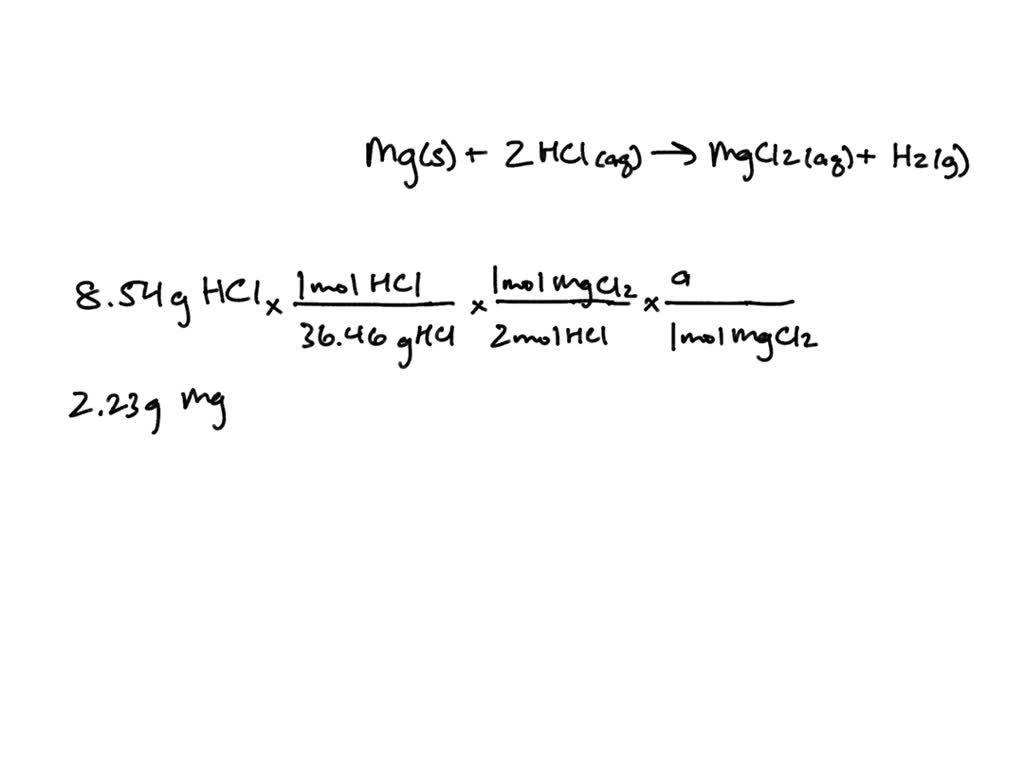
SOLVED Solid magnesium reacts with hydrochloric acid (HCI) to form
What Happens When Gold Reacts With Hydrochloric Acid When metals react with acids, a salt of the metal and hydrogen gas are formed. Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1. But when \muriatic acid is This page shows how the position of a metal in the reactivity series affects its reactions with common dilute acids such as hydrochloric acid. It is produced when sodium reacts with hydrochloric acid. When metals react with acids, a salt of the metal and hydrogen gas are formed. When gold is subjected to treatment with muriatic acid alone, nothing happens. For example, zinc reacts with hydro chloric acid to produce zinc chloride. Reactions of metals with acid. Reaction of gold with acids. Gold does not dissolve in nitric acid, hno 3 [4]. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of.
From www.nagwa.com
Question Video Determining the Name of the Salt Produced When Sodium What Happens When Gold Reacts With Hydrochloric Acid Reactions of metals with acid. Reaction of gold with acids. But when \muriatic acid is You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen. It is produced when sodium reacts with hydrochloric acid. Gold does. What Happens When Gold Reacts With Hydrochloric Acid.
From brainly.in
zinc reacts with dilute hydrochloric acid to give zinc chloride and What Happens When Gold Reacts With Hydrochloric Acid When gold is subjected to treatment with muriatic acid alone, nothing happens. Reactions of metals with acid. But when \muriatic acid is Gold does not dissolve in nitric acid, hno 3 [4]. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. When metals react with acids, a salt of the metal and hydrogen gas are. What Happens When Gold Reacts With Hydrochloric Acid.
From www.nagwa.com
Question Video Identifying Which Metals Form a Salt When Reacted with What Happens When Gold Reacts With Hydrochloric Acid Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen. Gold does not dissolve in nitric acid, hno 3 [4]. This page. What Happens When Gold Reacts With Hydrochloric Acid.
From blog.thepipingmart.com
What Happens when Aluminium Reacts with Hydrochloric acid What Happens When Gold Reacts With Hydrochloric Acid When gold is subjected to treatment with muriatic acid alone, nothing happens. Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1. Gold does not dissolve in nitric acid, hno 3 [4]. This page shows how the position of a metal in the reactivity. What Happens When Gold Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED When solid calcium hydroxide reacts with hydrochloric acid in What Happens When Gold Reacts With Hydrochloric Acid When metals react with acids, a salt of the metal and hydrogen gas are formed. Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1. Gold does not dissolve in nitric acid, hno 3 [4]. You have seen that in the reactions between metals. What Happens When Gold Reacts With Hydrochloric Acid.
From skylargokecox.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid What Happens When Gold Reacts With Hydrochloric Acid This page shows how the position of a metal in the reactivity series affects its reactions with common dilute acids such as hydrochloric acid. When gold is subjected to treatment with muriatic acid alone, nothing happens. When metals react with acids, a salt of the metal and hydrogen gas are formed. Au (s) + hno 3 (aq) gold dissolves in. What Happens When Gold Reacts With Hydrochloric Acid.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro What Happens When Gold Reacts With Hydrochloric Acid Reaction of gold with acids. When gold is subjected to treatment with muriatic acid alone, nothing happens. It is produced when sodium reacts with hydrochloric acid. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen.. What Happens When Gold Reacts With Hydrochloric Acid.
From www.teachoo.com
Metal compound A reacts with dilute hydrochloric acid to produce What Happens When Gold Reacts With Hydrochloric Acid It is produced when sodium reacts with hydrochloric acid. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. Gold does not dissolve in nitric acid, hno 3 [4]. Reactions of metals with acid. But when \muriatic acid is Reaction of gold with acids. When gold is subjected to treatment with muriatic acid alone, nothing happens.. What Happens When Gold Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED When an aqueous solution of hydrochloric acid is mixed with What Happens When Gold Reacts With Hydrochloric Acid It is produced when sodium reacts with hydrochloric acid. But when \muriatic acid is When gold is subjected to treatment with muriatic acid alone, nothing happens. Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1. Au (s) + hno 3 (aq) gold dissolves. What Happens When Gold Reacts With Hydrochloric Acid.
From www.vrogue.co
Sodium Metal Reacts With Hydrochloric Acid vrogue.co What Happens When Gold Reacts With Hydrochloric Acid For example, zinc reacts with hydro chloric acid to produce zinc chloride. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. This page shows how the position of a metal in the reactivity series affects its reactions with common dilute acids such as hydrochloric acid. Gold does not dissolve in nitric acid, hno 3 [4].. What Happens When Gold Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED When hydrochloric acid reacts with magnesium metal, hydrogen What Happens When Gold Reacts With Hydrochloric Acid Reactions of metals with acid. When metals react with acids, a salt of the metal and hydrogen gas are formed. Gold does not dissolve in nitric acid, hno 3 [4]. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions. What Happens When Gold Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED What is the balanced equation and the net ionic equation that What Happens When Gold Reacts With Hydrochloric Acid When gold is subjected to treatment with muriatic acid alone, nothing happens. It is produced when sodium reacts with hydrochloric acid. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a. What Happens When Gold Reacts With Hydrochloric Acid.
From www.youtube.com
What Happens when Magnesium reacts with Acetic Acid And Dilute What Happens When Gold Reacts With Hydrochloric Acid When gold is subjected to treatment with muriatic acid alone, nothing happens. Gold does not dissolve in nitric acid, hno 3 [4]. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. Reactions of metals with acid. Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated. What Happens When Gold Reacts With Hydrochloric Acid.
From www.coursehero.com
[Solved] Al reacts with hydrochloric acid according to the following What Happens When Gold Reacts With Hydrochloric Acid When metals react with acids, a salt of the metal and hydrogen gas are formed. Gold does not dissolve in nitric acid, hno 3 [4]. Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1. But when \muriatic acid is Reactions of metals with. What Happens When Gold Reacts With Hydrochloric Acid.
From www.academia.edu
(DOC) What happens if calcium carbonate reacts with hydrochloric acid What Happens When Gold Reacts With Hydrochloric Acid Reactions of metals with acid. When metals react with acids, a salt of the metal and hydrogen gas are formed. Reaction of gold with acids. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen. Au. What Happens When Gold Reacts With Hydrochloric Acid.
From www.chegg.com
Solved Gold reacts with nitric acid and hydrochloric acid What Happens When Gold Reacts With Hydrochloric Acid Gold does not dissolve in nitric acid, hno 3 [4]. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen. Reactions of metals with acid. When gold is subjected to treatment with muriatic acid alone, nothing. What Happens When Gold Reacts With Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science What Happens When Gold Reacts With Hydrochloric Acid But when \muriatic acid is This page shows how the position of a metal in the reactivity series affects its reactions with common dilute acids such as hydrochloric acid. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. Reactions of metals with acid. Reaction of gold with acids. It is produced when sodium reacts with. What Happens When Gold Reacts With Hydrochloric Acid.
From askfilo.com
Zinc metal reacts with hydrochloric acid by the following reaction Zn(s).. What Happens When Gold Reacts With Hydrochloric Acid But when \muriatic acid is You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen. Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid,. What Happens When Gold Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Solid magnesium reacts with hydrochloric acid (HCI) to form What Happens When Gold Reacts With Hydrochloric Acid But when \muriatic acid is For example, zinc reacts with hydro chloric acid to produce zinc chloride. Reaction of gold with acids. This page shows how the position of a metal in the reactivity series affects its reactions with common dilute acids such as hydrochloric acid. When gold is subjected to treatment with muriatic acid alone, nothing happens. Gold does. What Happens When Gold Reacts With Hydrochloric Acid.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo What Happens When Gold Reacts With Hydrochloric Acid Reaction of gold with acids. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. Reactions of metals with acid. Gold does. What Happens When Gold Reacts With Hydrochloric Acid.
From www.nagwa.com
Question Video Identifying the Products When a Metal Oxide Reacts with What Happens When Gold Reacts With Hydrochloric Acid For example, zinc reacts with hydro chloric acid to produce zinc chloride. It is produced when sodium reacts with hydrochloric acid. Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1. This page shows how the position of a metal in the reactivity series. What Happens When Gold Reacts With Hydrochloric Acid.
From skylargokecox.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid What Happens When Gold Reacts With Hydrochloric Acid Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1. When gold is subjected to treatment with muriatic acid alone, nothing happens. For example, zinc reacts with hydro chloric acid to produce zinc chloride. It is produced when sodium reacts with hydrochloric acid. Gold. What Happens When Gold Reacts With Hydrochloric Acid.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube What Happens When Gold Reacts With Hydrochloric Acid Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1. For example, zinc reacts with hydro chloric acid to produce zinc chloride. This page shows how the position of a metal in the reactivity series affects its reactions with common dilute acids such as. What Happens When Gold Reacts With Hydrochloric Acid.
From www.youtube.com
Q.11 What happens when Dilute Hydrochloric acid is added to iron What Happens When Gold Reacts With Hydrochloric Acid Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1. Gold does not dissolve in nitric acid, hno 3 [4]. Reaction of gold with acids. For example, zinc reacts with. What Happens When Gold Reacts With Hydrochloric Acid.
From www.vrogue.co
Sodium Carbonate Reacts With Hydrochloric Acid Balanc vrogue.co What Happens When Gold Reacts With Hydrochloric Acid But when \muriatic acid is It is produced when sodium reacts with hydrochloric acid. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen. Reactions of metals with acid. Reaction of gold with acids gold metal. What Happens When Gold Reacts With Hydrochloric Acid.
From savanahminwells.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid SavanahminWells What Happens When Gold Reacts With Hydrochloric Acid But when \muriatic acid is When gold is subjected to treatment with muriatic acid alone, nothing happens. Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1. This page shows how the position of a metal in the reactivity series affects its reactions with. What Happens When Gold Reacts With Hydrochloric Acid.
From mavink.com
Sodium Metal Reacts With Hydrochloric Acid What Happens When Gold Reacts With Hydrochloric Acid It is produced when sodium reacts with hydrochloric acid. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. When gold is subjected to treatment with muriatic acid alone, nothing happens. For example, zinc reacts with hydro chloric acid to produce zinc chloride. When metals react with acids, a salt of the metal and hydrogen gas. What Happens When Gold Reacts With Hydrochloric Acid.
From mavink.com
Sodium Metal Reacts With Hydrochloric Acid What Happens When Gold Reacts With Hydrochloric Acid When metals react with acids, a salt of the metal and hydrogen gas are formed. But when \muriatic acid is You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen. Reaction of gold with acids. It. What Happens When Gold Reacts With Hydrochloric Acid.
From savanahminwells.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid SavanahminWells What Happens When Gold Reacts With Hydrochloric Acid Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. It is produced when sodium reacts with hydrochloric acid. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and the hydrogen ions are reduced to hydrogen. Reaction of gold. What Happens When Gold Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Sodium carbonate reacts with hydrochloric acid to form sodium What Happens When Gold Reacts With Hydrochloric Acid When gold is subjected to treatment with muriatic acid alone, nothing happens. This page shows how the position of a metal in the reactivity series affects its reactions with common dilute acids such as hydrochloric acid. When metals react with acids, a salt of the metal and hydrogen gas are formed. You have seen that in the reactions between metals. What Happens When Gold Reacts With Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc gold hydrochloric acid What Happens When Gold Reacts With Hydrochloric Acid When metals react with acids, a salt of the metal and hydrogen gas are formed. Reaction of gold with acids. But when \muriatic acid is Reaction of gold with acids gold metal dissolves in aqua regia, a mixture of hydrochloric acid, hcl, and concentrated nitric acid, hno 3, in a 3:1. Reactions of metals with acid. For example, zinc reacts. What Happens When Gold Reacts With Hydrochloric Acid.
From askfilo.com
(4) When ferric oxide reacts with diluted hydrochloric acid. Ans. When Fe.. What Happens When Gold Reacts With Hydrochloric Acid Gold does not dissolve in nitric acid, hno 3 [4]. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. It is produced when sodium reacts with hydrochloric acid. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in the acid, and. What Happens When Gold Reacts With Hydrochloric Acid.
From byjus.com
11. What will be the product when 4 hydroxy benzyl alcohol react with What Happens When Gold Reacts With Hydrochloric Acid When metals react with acids, a salt of the metal and hydrogen gas are formed. When gold is subjected to treatment with muriatic acid alone, nothing happens. This page shows how the position of a metal in the reactivity series affects its reactions with common dilute acids such as hydrochloric acid. Reaction of gold with acids gold metal dissolves in. What Happens When Gold Reacts With Hydrochloric Acid.
From www.nagwa.com
Question Video Identifying the Name of the Gas Produced When Magnesium What Happens When Gold Reacts With Hydrochloric Acid For example, zinc reacts with hydro chloric acid to produce zinc chloride. When metals react with acids, a salt of the metal and hydrogen gas are formed. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a mixture of. But when \muriatic acid is Reaction of gold with acids. Gold does not dissolve in nitric acid, hno 3. What Happens When Gold Reacts With Hydrochloric Acid.
From brainly.in
What are the gases liberated when the dilute hydrochloric acid react What Happens When Gold Reacts With Hydrochloric Acid When metals react with acids, a salt of the metal and hydrogen gas are formed. For example, zinc reacts with hydro chloric acid to produce zinc chloride. It is produced when sodium reacts with hydrochloric acid. You have seen that in the reactions between metals and dilute acids, the metal is oxidised to metal ions by the hydrogen ions in. What Happens When Gold Reacts With Hydrochloric Acid.