What Is Medical Device Regulatory Affairs . The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Medical device companies must create and submit reports to both. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Explain fda’s role in regulating medical devices. This article explores the regulatory compliance and approval processes necessary for medical devices, focusing on various facets. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. Define a medical device and review basics about device classification. Describe five steps to get. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution.
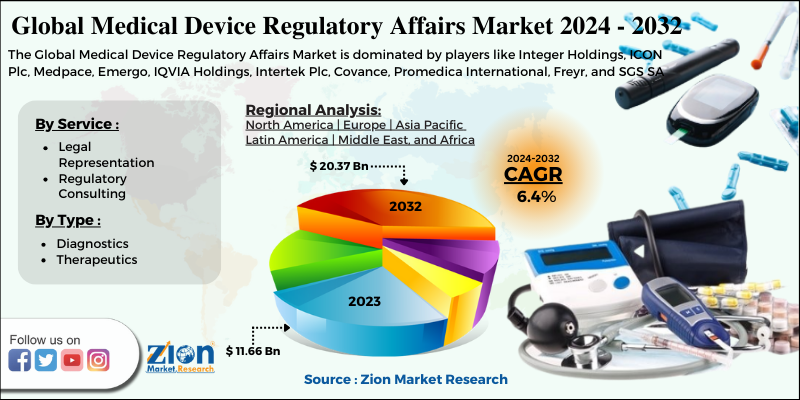
from www.zionmarketresearch.com
This article explores the regulatory compliance and approval processes necessary for medical devices, focusing on various facets. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. Medical device companies must create and submit reports to both. Describe five steps to get. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. Define a medical device and review basics about device classification. Explain fda’s role in regulating medical devices. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution.
Medical Device Regulatory Affairs Market Size, Share, Growth, Forecast 2028
What Is Medical Device Regulatory Affairs Define a medical device and review basics about device classification. Explain fda’s role in regulating medical devices. Define a medical device and review basics about device classification. This article explores the regulatory compliance and approval processes necessary for medical devices, focusing on various facets. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. Describe five steps to get. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical device companies must create and submit reports to both.
From www.qps.com
Global Regulatory Affairs QPS CustomBuilt Research What Is Medical Device Regulatory Affairs The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. Define a medical device and review basics about device classification. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical device companies must create and submit reports to. What Is Medical Device Regulatory Affairs.
From www.zionmarketresearch.com
Medical Device Regulatory Affairs Market Size, Share, Growth, Forecast 2028 What Is Medical Device Regulatory Affairs As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. Describe five steps to get. Define a medical device and review basics about device classification. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. Explain fda’s role. What Is Medical Device Regulatory Affairs.
From www.qps.com
Global Regulatory Affairs QPS CustomBuilt Research What Is Medical Device Regulatory Affairs Medical device companies must create and submit reports to both. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. Describe five steps to get. Explain fda’s role in regulating medical devices. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that. What Is Medical Device Regulatory Affairs.
From www.researchgate.net
Roles of Regulatory affair department Download Scientific Diagram What Is Medical Device Regulatory Affairs Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. Medical device companies must create and submit reports to both. Explain fda’s role in regulating medical devices. The basic regulatory requirements that manufacturers. What Is Medical Device Regulatory Affairs.
From 4usconsulting.pt
Medical Affairs e Regulatory Affairs Evolução de Mercado — 4 US CONSULTING What Is Medical Device Regulatory Affairs Medical device companies must create and submit reports to both. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. This article explores the regulatory compliance and approval processes necessary for medical devices, focusing on various facets. The objective of this paper is to provide an introduction and overview of. What Is Medical Device Regulatory Affairs.
From www.slideshare.net
Medical Device Regulatory Affairs. PPT What Is Medical Device Regulatory Affairs As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. Explain fda’s role in regulating medical devices. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Describe five steps to get. Medical device companies must create and submit reports to. What Is Medical Device Regulatory Affairs.
From www.heartlungcirc.org
Regulatory Requirements For Medical Devices And Vascular Ageing An What Is Medical Device Regulatory Affairs The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. The objective of this paper is to provide an introduction and. What Is Medical Device Regulatory Affairs.
From www.medicalaffairsspecialist.org
Home ACMA What Is Medical Device Regulatory Affairs Medical device companies must create and submit reports to both. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. Describe five steps to get. As the fda center responsible for the regulatory oversight. What Is Medical Device Regulatory Affairs.
From www.scribd.com
Handbook of Medical Device Regulatory Affairs in Asia Medical Device What Is Medical Device Regulatory Affairs Medical device companies must create and submit reports to both. Explain fda’s role in regulating medical devices. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. As the fda center responsible for the. What Is Medical Device Regulatory Affairs.
From operonstrategist.com
Learn Professionally Quality and Regulatory Affairs of Medical Device What Is Medical Device Regulatory Affairs This article explores the regulatory compliance and approval processes necessary for medical devices, focusing on various facets. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. Describe five steps to get. Medical device companies must create and submit reports to both. As the fda center responsible for. What Is Medical Device Regulatory Affairs.
From markwideresearch.com
Europe Medical Device Regulatory Affairs Outsourcing market 20242032 What Is Medical Device Regulatory Affairs Explain fda’s role in regulating medical devices. Medical device companies must create and submit reports to both. Define a medical device and review basics about device classification. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. Describe five steps to get. The basic regulatory requirements that manufacturers. What Is Medical Device Regulatory Affairs.
From qbdgroup.com
The key role of Regulatory Affairs in the pharmaceutical industry What Is Medical Device Regulatory Affairs Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Define a medical device and review basics about device classification. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device.. What Is Medical Device Regulatory Affairs.
From www.ppd.com
‘Right First Time’ Approach to PostAuthorization Strategy PPD Inc What Is Medical Device Regulatory Affairs Explain fda’s role in regulating medical devices. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. Medical device companies must create and submit reports to both. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Medical device regulation (mdr) spans the product life cycle of. What Is Medical Device Regulatory Affairs.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I What Is Medical Device Regulatory Affairs As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. Define a medical device and. What Is Medical Device Regulatory Affairs.
From medicalqms.com.au
News and Updates • Medical Devices QMS and Regulatory Affairs Melbourne What Is Medical Device Regulatory Affairs The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Define a medical device and review basics about device classification. Describe five steps to get. Medical device companies must create and submit reports to both. This article explores. What Is Medical Device Regulatory Affairs.
From zivadra.com
Demystifying Regulatory Affairs in Medical Devices A Comprehensive What Is Medical Device Regulatory Affairs Define a medical device and review basics about device classification. This article explores the regulatory compliance and approval processes necessary for medical devices, focusing on various facets. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Explain. What Is Medical Device Regulatory Affairs.
From www.slideshare.net
Medical Device Regulatory Affairs. What Is Medical Device Regulatory Affairs As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. Describe five steps to get. This article explores the regulatory. What Is Medical Device Regulatory Affairs.
From www.cyient.com
Whitepaper Overview of Medical Device Regulatory Affairs What Is Medical Device Regulatory Affairs Medical device companies must create and submit reports to both. Describe five steps to get. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. Explain fda’s role. What Is Medical Device Regulatory Affairs.
From www.insightaceanalytic.com
Medical Device Regulatory Affairs Market Size, Scope and Demand Analysis What Is Medical Device Regulatory Affairs Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. This article explores the regulatory compliance and approval processes necessary for medical devices, focusing on various facets. As the fda center. What Is Medical Device Regulatory Affairs.
From www.researchandmarkets.com
Medical Device Regulatory Affairs Market Size, Share & Trends Analysis What Is Medical Device Regulatory Affairs The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. This article explores the regulatory compliance and approval processes necessary for. What Is Medical Device Regulatory Affairs.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is Medical Device Regulatory Affairs Medical device companies must create and submit reports to both. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. This article explores the regulatory compliance and approval processes necessary for medical devices, focusing on various facets.. What Is Medical Device Regulatory Affairs.
From www.researchandmarkets.com
Medical Device Regulatory Affairs Market Size, Share & Trends Analysis What Is Medical Device Regulatory Affairs The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. Medical device companies must create and submit reports to both. Explain fda’s role in regulating medical devices. Medical. What Is Medical Device Regulatory Affairs.
From template.mapadapalavra.ba.gov.br
Medical Device Regulatory Strategy Template What Is Medical Device Regulatory Affairs Define a medical device and review basics about device classification. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Describe five steps to get. This article explores the regulatory compliance and approval processes necessary for medical devices, focusing on various facets. The objective of this paper is to provide an. What Is Medical Device Regulatory Affairs.
From www.slideshare.net
Medical Device Regulatory Affairs. PPT What Is Medical Device Regulatory Affairs As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. The regulations on medical devices (regulation (eu) 2017/745) and on. What Is Medical Device Regulatory Affairs.
From viseven.com
Pharmaceutical Medical Affairs Mastery Your Ultimate Guide What Is Medical Device Regulatory Affairs Describe five steps to get. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. Explain fda’s role in regulating medical devices. As the fda center responsible for the regulatory oversight of medical devices, cdrh. What Is Medical Device Regulatory Affairs.
From royed.in
US Medical Device Regulatory Affairs Royed Training What Is Medical Device Regulatory Affairs Medical device companies must create and submit reports to both. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays. What Is Medical Device Regulatory Affairs.
From www.insightaceanalytic.com
Medical Device Regulatory Affairs Market Size, Scope and Demand Analysis What Is Medical Device Regulatory Affairs Describe five steps to get. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. Explain fda’s role in regulating medical devices. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that. What Is Medical Device Regulatory Affairs.
From imgbin.com
Regulatory Affairs Medical Device Regulatory Compliance Medicine What Is Medical Device Regulatory Affairs This article explores the regulatory compliance and approval processes necessary for medical devices, focusing on various facets. Define a medical device and review basics about device classification. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. Explain fda’s role in regulating medical devices. The regulations on medical devices (regulation (eu) 2017/745) and. What Is Medical Device Regulatory Affairs.
From www.i-pharmconsulting.com
Insights on the Medical Device Regulatory Affairs Global Market to 2031 What Is Medical Device Regulatory Affairs Medical device companies must create and submit reports to both. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. Define a medical device and review basics about device classification. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in. What Is Medical Device Regulatory Affairs.
From mdphysicianmag.com
Medical Device Regulatory Affairs Training Courses Lead by Industry What Is Medical Device Regulatory Affairs As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. Describe five steps to get. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. This article explores the regulatory compliance and approval processes necessary for medical devices,. What Is Medical Device Regulatory Affairs.
From www.sketchbubble.com
Regulatory Affairs PowerPoint and Google Slides Template PPT Slides What Is Medical Device Regulatory Affairs The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. Define a medical device and review basics about device classification. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. The basic regulatory requirements that manufacturers of medical devices distributed in. What Is Medical Device Regulatory Affairs.
From betebt.com
Medical Device Regulation Importance and Examples in APAC (2022) What Is Medical Device Regulatory Affairs Medical device companies must create and submit reports to both. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. Describe five steps to get. Define a medical device and review basics about device classification. This article explores the regulatory compliance and approval processes necessary for medical devices, focusing on. What Is Medical Device Regulatory Affairs.
From www.indiamart.com
Medical Device Regulatory Affairs Service at best price in Ahmedabad What Is Medical Device Regulatory Affairs Define a medical device and review basics about device classification. As the fda center responsible for the regulatory oversight of medical devices, cdrh plays a crucial role in facilitating device. Explain fda’s role in regulating medical devices. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Describe five steps to. What Is Medical Device Regulatory Affairs.
From www.fiverr.com
Assist in regulatory affairs specialist for medical, pharma and ip What Is Medical Device Regulatory Affairs Describe five steps to get. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. Explain fda’s role in regulating medical devices. As the fda center responsible for the regulatory oversight. What Is Medical Device Regulatory Affairs.
From cm-plus.vn
Pharmaceutical Regulatory Affairs Consulting CM PLUS VIETNAM What Is Medical Device Regulatory Affairs Define a medical device and review basics about device classification. The objective of this paper is to provide an introduction and overview of regulatory affairs for manufacturers that are new to the. Medical device regulation (mdr) spans the product life cycle of a medical device from discovery to distribution. The basic regulatory requirements that manufacturers of medical devices distributed in. What Is Medical Device Regulatory Affairs.