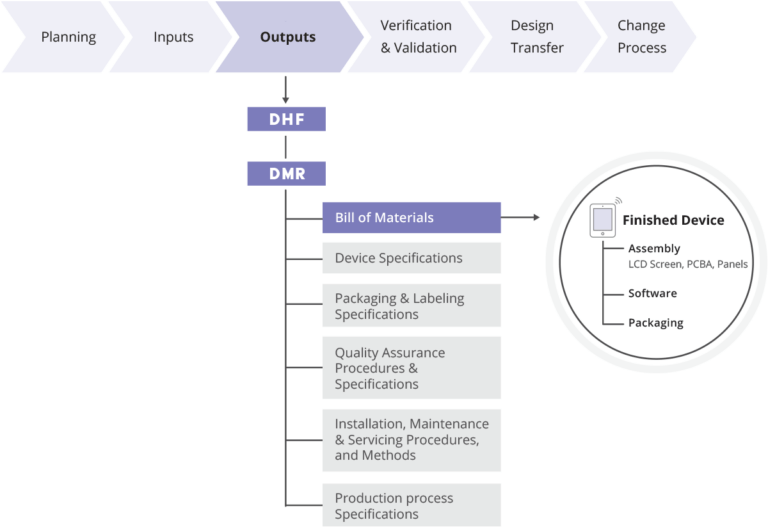
Device Master Record Definition Fda . each manufacturer shall maintain device master records (dmr's). device master files. § 820.181 device master record. Introduction to master files for devices (mafs) a premarket approval application. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. Each manufacturer shall maintain device master records (dmr's). § 820.181 device master record. (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. Identify key definitions related to documents and records. Each manufacturer shall maintain device master records (dmr's). Each manufacturer shall ensure that each.
from www.arenasolutions.com
§ 820.181 device master record. each manufacturer shall maintain device master records (dmr's). Identify key definitions related to documents and records. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). Each manufacturer shall ensure that each. (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. device master files. Introduction to master files for devices (mafs) a premarket approval application. Each manufacturer shall maintain device master records (dmr's).
Device Master Record (DMR) Definition Arena
Device Master Record Definition Fda (i) device history record (dhr) means a compilation of records containing the production history of a finished device. device master files. Each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain device master records (dmr's). (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. § 820.181 device master record. each manufacturer shall maintain device master records (dmr's). Introduction to master files for devices (mafs) a premarket approval application. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. § 820.181 device master record. Each manufacturer shall ensure that each. Identify key definitions related to documents and records.
From www.technia.co.jp
What is a Device Master Record? TECHNIA (Japan) Device Master Record Definition Fda Each manufacturer shall ensure that each. § 820.181 device master record. Introduction to master files for devices (mafs) a premarket approval application. device master files. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. Identify key definitions related to documents and records. (j) device master record (dmr). Device Master Record Definition Fda.
From www.arenasolutions.com
Device Master Record (DMR) Definition Arena Device Master Record Definition Fda Each manufacturer shall ensure that each. (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. device master files. Each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain device master records (dmr's). § 820.181 device master record. each manufacturer shall maintain device master records (dmr's). . Device Master Record Definition Fda.
From www.dvelop-ls.de
Device Master Record (DMR) Erklärung im Glossar lesen Device Master Record Definition Fda § 820.181 device master record. Each manufacturer shall ensure that each. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. Identify key definitions related to documents and records. Each manufacturer shall maintain device master records (dmr's). Introduction to master files for devices (mafs) a premarket approval application. each. Device Master Record Definition Fda.
From www.bizmanualz.com
Device Master Record Procedure Template Word Device Master Record Definition Fda Each manufacturer shall maintain device master records (dmr's). Each manufacturer shall ensure that each. (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. Each manufacturer shall maintain device master records (dmr's). Introduction. Device Master Record Definition Fda.
From www.qualitymeddev.com
Device Master Record Overview of FDA Requiements Device Master Record Definition Fda § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). Introduction to master files for devices (mafs) a premarket approval application. device master files. § 820.181 device master record. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. (j) device master record (dmr). Device Master Record Definition Fda.
From www.bizmanualz.com
Device Master Record Contents Template Word Device Master Record Definition Fda Identify key definitions related to documents and records. Each manufacturer shall ensure that each. § 820.181 device master record. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. Each manufacturer shall maintain device master records (dmr's). Introduction to master files for devices (mafs) a premarket approval application. Each manufacturer. Device Master Record Definition Fda.
From www.bizmanualz.com
Device Master Record Procedure Template Word Device Master Record Definition Fda each manufacturer shall maintain device master records (dmr's). (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. § 820.181 device master record. Identify key definitions related to documents and records. Each manufacturer shall maintain device master records (dmr's). § 820.181 device master record. (i) device history record. Device Master Record Definition Fda.
From www.slideserve.com
PPT Design documentation PowerPoint Presentation ID1625484 Device Master Record Definition Fda Each manufacturer shall maintain device master records (dmr's). § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). § 820.181 device master record. (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. Identify key definitions related to documents and records. device master files. Each manufacturer. Device Master Record Definition Fda.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID Device Master Record Definition Fda Each manufacturer shall ensure that each. (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. Identify key definitions related to documents and records. Each manufacturer shall maintain device master records (dmr's). Introduction. Device Master Record Definition Fda.
From old.sermitsiaq.ag
Device History Record Template Device Master Record Definition Fda (i) device history record (dhr) means a compilation of records containing the production history of a finished device. Introduction to master files for devices (mafs) a premarket approval application. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). Each manufacturer shall ensure that each. (j) device master record (dmr) means a compilation of. Device Master Record Definition Fda.
From dokumen.tips
(PDF) 2015 Annual FDA Medical Device Quality System Data Device Master Record Definition Fda § 820.181 device master record. Identify key definitions related to documents and records. each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain device master records (dmr's). § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). device master files. (j) device master record (dmr) means a compilation of records. Device Master Record Definition Fda.
From www.johner-institut.de
Device Master Record DMR Auch für Software?!? Device Master Record Definition Fda § 820.181 device master record. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. Each manufacturer shall ensure that each. device master files. Identify key definitions related to documents and records. each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain device master records (dmr's).. Device Master Record Definition Fda.
From www.vrogue.co
Device Master Records Design History Files Gmp Docs vrogue.co Device Master Record Definition Fda Each manufacturer shall ensure that each. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain device master records (dmr's). Identify key definitions related to documents and records. device master files. § 820.181 device master record.. Device Master Record Definition Fda.
From www.qualitymeddev.com
Device Master Record Overview of FDA Requiements Device Master Record Definition Fda (i) device history record (dhr) means a compilation of records containing the production history of a finished device. § 820.181 device master record. Identify key definitions related to documents and records. § 820.181 device master record. Introduction to master files for devices (mafs) a premarket approval application. Each manufacturer shall maintain device master records (dmr's). Each manufacturer. Device Master Record Definition Fda.
From www.technia.co.uk
What is a Device Master Record? TECHNIA (UK) Device Master Record Definition Fda § 820.181 device master record. Identify key definitions related to documents and records. Each manufacturer shall maintain device master records (dmr's). Each manufacturer shall ensure that each. Each manufacturer shall maintain device master records (dmr's). (i) device history record (dhr) means a compilation of records containing the production history of a finished device. (j) device master record. Device Master Record Definition Fda.
From www.biobostonconsulting.com
Crucial Components of the Device Master Record (DMR) for Medical Device Device Master Record Definition Fda Each manufacturer shall maintain device master records (dmr's). (i) device history record (dhr) means a compilation of records containing the production history of a finished device. § 820.181 device master record. Each manufacturer shall ensure that each. § 820.181 device master record. (j) device master record (dmr) means a compilation of records containing the procedures and. Device Master Record Definition Fda.
From www.scribd.com
Device Master Records.doc Specification (Technical Standard Device Master Record Definition Fda Introduction to master files for devices (mafs) a premarket approval application. Each manufacturer shall maintain device master records (dmr's). device master files. § 820.181 device master record. (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. Identify key definitions related to documents and records. § 820.181 device master. Device Master Record Definition Fda.
From www.qualitymeddev.com
Device Master Record Overview of FDA Requiements Device Master Record Definition Fda Each manufacturer shall ensure that each. (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. Identify key definitions related to documents and records. § 820.181 device master record. each manufacturer shall maintain device master records (dmr's). device master files. (i) device history record (dhr) means a compilation. Device Master Record Definition Fda.
From studylib.net
Device Master Record Device Master Record Definition Fda § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). device master files. Introduction to master files for devices (mafs) a premarket approval application. each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain device master records (dmr's). (i) device history record (dhr) means a compilation of records containing the production. Device Master Record Definition Fda.
From www.instantgmp.com
Device Master Record Device Master Record Definition Fda Introduction to master files for devices (mafs) a premarket approval application. § 820.181 device master record. § 820.181 device master record. Identify key definitions related to documents and records. Each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain device master records (dmr's). each manufacturer shall maintain device master records (dmr's). (j) device master. Device Master Record Definition Fda.
From www.instantgmp.com
Device Master Record Device Master Record Definition Fda Identify key definitions related to documents and records. § 820.181 device master record. § 820.181 device master record. each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain device master records (dmr's). (i) device history record (dhr) means a compilation of records containing the production history of a finished device. Each manufacturer shall ensure. Device Master Record Definition Fda.
From dokumen.tips
(PDF) DMRDevice Master Record vs DHFDesign History · PDF Device Master Record Definition Fda Introduction to master files for devices (mafs) a premarket approval application. each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain device master records (dmr's). (i) device history record (dhr) means a compilation of records containing the production history of a finished device. Each manufacturer shall maintain device master records (dmr's). § 820.181 device master. Device Master Record Definition Fda.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device Device Master Record Definition Fda Each manufacturer shall maintain device master records (dmr's). § 820.181 device master record. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. Each manufacturer shall ensure that each. § 820.181. Device Master Record Definition Fda.
From www.presentationeze.com
Device Master Record (DMR) What needs to be recorded into the DMR Device Master Record Definition Fda Identify key definitions related to documents and records. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. each manufacturer shall maintain device master records (dmr's). Each manufacturer shall ensure that each. § 820.181 device master record. § 820.181 device master record. Each manufacturer shall maintain device master. Device Master Record Definition Fda.
From studylib.net
(DHF), Device Master Record (DMR), Device History Device Master Record Definition Fda Identify key definitions related to documents and records. § 820.181 device master record. each manufacturer shall maintain device master records (dmr's). Each manufacturer shall ensure that each. device master files. Each manufacturer shall maintain device master records (dmr's). (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. . Device Master Record Definition Fda.
From www.instantgmp.com
Device Master Record Device Master Record Definition Fda Each manufacturer shall maintain device master records (dmr's). (i) device history record (dhr) means a compilation of records containing the production history of a finished device. each manufacturer shall maintain device master records (dmr's). device master files. § 820.181 device master record. § 820.181 device master record. Identify key definitions related to documents and records.. Device Master Record Definition Fda.
From fdacompliancehelp.blogspot.com
FDA Compliance Help October 2011 Device Master Record Definition Fda Introduction to master files for devices (mafs) a premarket approval application. Each manufacturer shall ensure that each. Each manufacturer shall maintain device master records (dmr's). each manufacturer shall maintain device master records (dmr's). Identify key definitions related to documents and records. Each manufacturer shall maintain device master records (dmr's). device master files. (j) device master record (dmr). Device Master Record Definition Fda.
From www.youtube.com
Design History File (DHF), the Device Master Record (DMR) and the Device Master Record Definition Fda Each manufacturer shall maintain device master records (dmr's). Identify key definitions related to documents and records. § 820.181 device master record. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain device master records (dmr's). Introduction to. Device Master Record Definition Fda.
From www.technia.com
What is a Device Master Record? TECHNIA Device Master Record Definition Fda each manufacturer shall maintain device master records (dmr's). device master files. Each manufacturer shall ensure that each. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). Identify key definitions related to documents and records. § 820.181 device master record. (j) device master record (dmr) means a compilation of records containing the. Device Master Record Definition Fda.
From www.presentationeze.com
FDA QSR's Handling, Storage, Distribution and Installation.PresentationEZE Device Master Record Definition Fda Each manufacturer shall ensure that each. device master files. Introduction to master files for devices (mafs) a premarket approval application. each manufacturer shall maintain device master records (dmr's). Identify key definitions related to documents and records. (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. § 820.181 device. Device Master Record Definition Fda.
From www.youtube.com
Preparing a Device Master Record (DMR) YouTube Device Master Record Definition Fda device master files. each manufacturer shall maintain device master records (dmr's). (i) device history record (dhr) means a compilation of records containing the production history of a finished device. Introduction to master files for devices (mafs) a premarket approval application. Each manufacturer shall maintain device master records (dmr's). § 820.181 device master record. § 820.181. Device Master Record Definition Fda.
From hardcoreqms.com
Device Master Records (DMR) for Medical Devices (2023) Device Master Record Definition Fda Introduction to master files for devices (mafs) a premarket approval application. Each manufacturer shall maintain device master records (dmr's). device master files. (j) device master record (dmr) means a compilation of records containing the procedures and specifications for a. § 820.181 device master record. each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain. Device Master Record Definition Fda.
From www.technia.us
What is a Device Master Record? TECHNIA (US) Device Master Record Definition Fda Identify key definitions related to documents and records. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. § 820.181 device master record. Introduction to master files for devices (mafs) a premarket approval application. (j) device master record (dmr) means a compilation of records containing the procedures and specifications. Device Master Record Definition Fda.
From slidetodoc.com
Design History File Device Master Record and Device Device Master Record Definition Fda Each manufacturer shall ensure that each. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). Introduction to master files for devices (mafs) a premarket approval application. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. each manufacturer shall maintain device master records (dmr's). . Device Master Record Definition Fda.
From www.youtube.com
Design History File DHF, Device Master Record DMR, Device History Device Master Record Definition Fda Each manufacturer shall maintain device master records (dmr's). Identify key definitions related to documents and records. § 820.181 device master record. device master files. (i) device history record (dhr) means a compilation of records containing the production history of a finished device. Introduction to master files for devices (mafs) a premarket approval application. Each manufacturer shall ensure. Device Master Record Definition Fda.