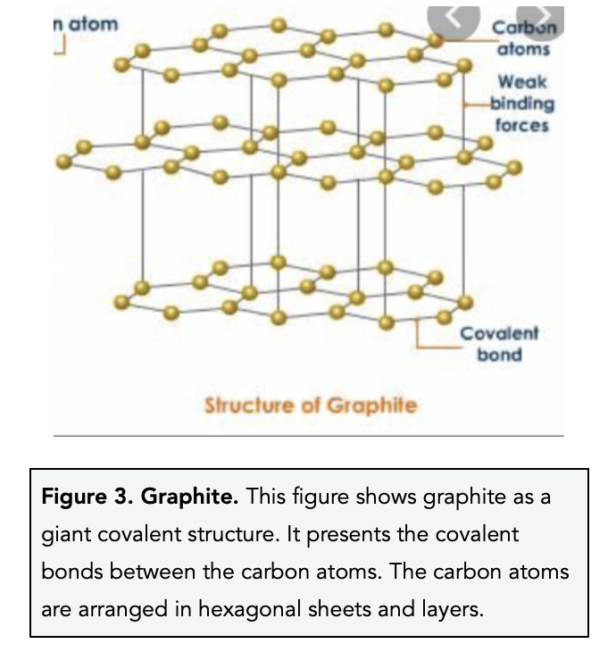
Which Is Not An Element Diamond Graphite Silica Ozone . (a) diamond (b) graphite (c) silica (d) ozone an element is identified by its atomic number not by anything else. silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is a compound, not an element. which one of the following is not an element? explain why diamond does not conduct electricity and why graphite does conduct electricity. silicon dioxide is known as silica with molecular formula = s i o 2 which is a compound, not an element. Diamond, graphite and graphene are forms of carbon. for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. giant covalent substances have many atoms joined together by covalent bonds. revision notes on diamond, graphite & silicon (iv) oxide for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$.
from studymind.co.uk
(a) diamond (b) graphite (c) silica (d) ozone which one of the following is not an element? for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is a compound, not an element. explain why diamond does not conduct electricity and why graphite does conduct electricity. giant covalent substances have many atoms joined together by covalent bonds. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. Diamond, graphite and graphene are forms of carbon. an element is identified by its atomic number not by anything else. silicon dioxide is known as silica with molecular formula = s i o 2 which is a compound, not an element.
Silicon Dioxide, Diamond & Graphite (GCSE Chemistry) Study Mind
Which Is Not An Element Diamond Graphite Silica Ozone an element is identified by its atomic number not by anything else. explain why diamond does not conduct electricity and why graphite does conduct electricity. revision notes on diamond, graphite & silicon (iv) oxide for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. silicon dioxide is known as silica with molecular formula = s i o 2 which is a compound, not an element. giant covalent substances have many atoms joined together by covalent bonds. an element is identified by its atomic number not by anything else. Diamond, graphite and graphene are forms of carbon. silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is a compound, not an element. (a) diamond (b) graphite (c) silica (d) ozone Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. which one of the following is not an element?
From www.thesciencehive.co.uk
Covalent bonding (GCSE) — the science sauce Which Is Not An Element Diamond Graphite Silica Ozone giant covalent substances have many atoms joined together by covalent bonds. explain why diamond does not conduct electricity and why graphite does conduct electricity. which one of the following is not an element? for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. (a) diamond (b) graphite. Which Is Not An Element Diamond Graphite Silica Ozone.
From studymind.co.uk
Silicon Dioxide, Diamond & Graphite (GCSE Chemistry) Study Mind Which Is Not An Element Diamond Graphite Silica Ozone which one of the following is not an element? Diamond, graphite and graphene are forms of carbon. giant covalent substances have many atoms joined together by covalent bonds. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.science-revision.co.uk
Diamond, graphite and silica Which Is Not An Element Diamond Graphite Silica Ozone giant covalent substances have many atoms joined together by covalent bonds. revision notes on diamond, graphite & silicon (iv) oxide for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. Diamond, graphite and graphene are forms of carbon. silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is. Which Is Not An Element Diamond Graphite Silica Ozone.
From physicsopenlab.org
Graphite Structure PhysicsOpenLab Which Is Not An Element Diamond Graphite Silica Ozone silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is a compound, not an element. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. an element is identified by its atomic number not by anything else. for example, knowing that silica (sio2) combines silicon and oxygen atoms. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.science-revision.co.uk
Diamond, graphite and silica Which Is Not An Element Diamond Graphite Silica Ozone an element is identified by its atomic number not by anything else. silicon dioxide is known as silica with molecular formula = s i o 2 which is a compound, not an element. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. which one of the following is not an. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.meritnation.com
1 Which of the following is not an element a Diamond b graphite c Which Is Not An Element Diamond Graphite Silica Ozone silicon dioxide is known as silica with molecular formula = s i o 2 which is a compound, not an element. Diamond, graphite and graphene are forms of carbon. an element is identified by its atomic number not by anything else. explain why diamond does not conduct electricity and why graphite does conduct electricity. revision notes. Which Is Not An Element Diamond Graphite Silica Ozone.
From askanearthspacescientist.asu.edu
Finding and Making Diamonds Ask An Earth and Space Scientist Which Is Not An Element Diamond Graphite Silica Ozone silicon dioxide is known as silica with molecular formula = s i o 2 which is a compound, not an element. (a) diamond (b) graphite (c) silica (d) ozone giant covalent substances have many atoms joined together by covalent bonds. explain why diamond does not conduct electricity and why graphite does conduct electricity. for example, knowing. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.slideserve.com
PPT Structure of Minerals PowerPoint Presentation, free download ID Which Is Not An Element Diamond Graphite Silica Ozone (a) diamond (b) graphite (c) silica (d) ozone which one of the following is not an element? revision notes on diamond, graphite & silicon (iv) oxide for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$.. Which Is Not An Element Diamond Graphite Silica Ozone.
From ar.inspiredpencil.com
Diamond Structure Vs Graphite Structure Which Is Not An Element Diamond Graphite Silica Ozone giant covalent substances have many atoms joined together by covalent bonds. which one of the following is not an element? silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is a compound, not an element. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. silicon dioxide. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.youtube.com
The structures and properties of diamond, graphite and silica YouTube Which Is Not An Element Diamond Graphite Silica Ozone which one of the following is not an element? for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. (a) diamond (b) graphite (c) silica (d) ozone Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. silicon dioxide is known as. Which Is Not An Element Diamond Graphite Silica Ozone.
From studymind.co.uk
Silicon Dioxide, Diamond & Graphite (GCSE Chemistry) Study Mind Which Is Not An Element Diamond Graphite Silica Ozone silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is a compound, not an element. revision notes on diamond, graphite & silicon (iv) oxide for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ ,. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.slideserve.com
PPT Lecture Notes Chem 150 K. Marr PowerPoint Presentation ID697651 Which Is Not An Element Diamond Graphite Silica Ozone Diamond, graphite and graphene are forms of carbon. an element is identified by its atomic number not by anything else. which one of the following is not an element? Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals. Which Is Not An Element Diamond Graphite Silica Ozone.
From cezeabjz.blob.core.windows.net
Graphite Chemical Formula Name at Carol Hutchinson blog Which Is Not An Element Diamond Graphite Silica Ozone Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. explain why diamond does not conduct electricity and why graphite does conduct electricity. for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. silicon dioxide is known as silica with the molecular. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.youtube.com
Giant covalent structures diamond and graphite YouTube Which Is Not An Element Diamond Graphite Silica Ozone an element is identified by its atomic number not by anything else. for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. giant covalent substances have many atoms joined together by covalent bonds. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ ,. Which Is Not An Element Diamond Graphite Silica Ozone.
From studymind.co.uk
Silicon Dioxide, Diamond & Graphite (GCSE Chemistry) Study Mind Which Is Not An Element Diamond Graphite Silica Ozone Diamond, graphite and graphene are forms of carbon. an element is identified by its atomic number not by anything else. which one of the following is not an element? revision notes on diamond, graphite & silicon (iv) oxide for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. silicon dioxide. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.youtube.com
Diamond vs Graphite Quick differences and comparison YouTube Which Is Not An Element Diamond Graphite Silica Ozone Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. (a) diamond (b) graphite (c) silica (d) ozone revision notes on diamond, graphite & silicon (iv) oxide for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. which one of the following is not an element?. Which Is Not An Element Diamond Graphite Silica Ozone.
From askanydifference.com
Diamond vs Graphite Difference and Comparison Which Is Not An Element Diamond Graphite Silica Ozone Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is a compound, not an element. revision notes on diamond, graphite & silicon (iv) oxide for the cambridge o level chemistry syllabus, written by the chemistry experts at save my. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.researchgate.net
Periodic table of color centers in diamond. The table shows the most Which Is Not An Element Diamond Graphite Silica Ozone Diamond, graphite and graphene are forms of carbon. (a) diamond (b) graphite (c) silica (d) ozone silicon dioxide is known as silica with molecular formula = s i o 2 which is a compound, not an element. which one of the following is not an element? an element is identified by its atomic number not by anything. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.learnatnoon.com
How is graphite different from diamonds? Noon Academy Which Is Not An Element Diamond Graphite Silica Ozone Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. an element is identified by its atomic number not by anything else. silicon dioxide is known as silica with the molecular formula. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.meritnation.com
1 Which of the following is not an element a Diamond b graphite c Which Is Not An Element Diamond Graphite Silica Ozone an element is identified by its atomic number not by anything else. silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is a compound, not an element. for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. silicon dioxide is known as silica with. Which Is Not An Element Diamond Graphite Silica Ozone.
From ar.inspiredpencil.com
Structure Of Graphite And Diamond Which Is Not An Element Diamond Graphite Silica Ozone Diamond, graphite and graphene are forms of carbon. revision notes on diamond, graphite & silicon (iv) oxide for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. silicon dioxide is known as silica with molecular formula = s i o 2 which is a compound, not an element. an element is. Which Is Not An Element Diamond Graphite Silica Ozone.
From askanydifference.com
Diamond vs Graphite Difference and Comparison Which Is Not An Element Diamond Graphite Silica Ozone an element is identified by its atomic number not by anything else. explain why diamond does not conduct electricity and why graphite does conduct electricity. for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. giant covalent substances have many atoms joined together by covalent bonds. Element. Which Is Not An Element Diamond Graphite Silica Ozone.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Which Is Not An Element Diamond Graphite Silica Ozone explain why diamond does not conduct electricity and why graphite does conduct electricity. an element is identified by its atomic number not by anything else. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound,. Which Is Not An Element Diamond Graphite Silica Ozone.
From newatlas.com
Stanford scientists find a new way to turn graphite into diamond Which Is Not An Element Diamond Graphite Silica Ozone revision notes on diamond, graphite & silicon (iv) oxide for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. giant covalent substances have many atoms joined together by covalent bonds. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. explain why diamond does not. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.science-revision.co.uk
Diamond, graphite and silica Which Is Not An Element Diamond Graphite Silica Ozone giant covalent substances have many atoms joined together by covalent bonds. an element is identified by its atomic number not by anything else. silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is a compound, not an element. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$.. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.researchgate.net
Periodic table of color centers in diamond. The table shows the most Which Is Not An Element Diamond Graphite Silica Ozone an element is identified by its atomic number not by anything else. giant covalent substances have many atoms joined together by covalent bonds. Diamond, graphite and graphene are forms of carbon. silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is a compound, not an element. revision notes on diamond, graphite & silicon. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.amazon.co.uk
Mark Teaching Model Chemical Molecular Crystal Structure Model Diamond Which Is Not An Element Diamond Graphite Silica Ozone giant covalent substances have many atoms joined together by covalent bonds. silicon dioxide is known as silica with the molecular formula \[=si{{o}_{2}}\] which is a compound, not an element. (a) diamond (b) graphite (c) silica (d) ozone silicon dioxide is known as silica with molecular formula = s i o 2 which is a compound, not an. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.amazon.co.uk
LifeSize Teaching Model Chemical Molecular Crystal Structure Model Which Is Not An Element Diamond Graphite Silica Ozone an element is identified by its atomic number not by anything else. giant covalent substances have many atoms joined together by covalent bonds. for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ ,. Which Is Not An Element Diamond Graphite Silica Ozone.
From slideplayer.com
Covalent Properties Main Concept ppt download Which Is Not An Element Diamond Graphite Silica Ozone silicon dioxide is known as silica with molecular formula = s i o 2 which is a compound, not an element. Diamond, graphite and graphene are forms of carbon. for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. which one of the following is not an element?. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.researchgate.net
Fine spectrum of o element in diamond under different ozone Which Is Not An Element Diamond Graphite Silica Ozone for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. which one of the following is not an element? explain why diamond does not conduct electricity and why graphite does conduct electricity. Diamond, graphite and graphene are forms of carbon. silicon dioxide is known as silica with. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.scienceabc.com
Does The Difference In Structure Make Graphite Soft But Diamond Hard? Which Is Not An Element Diamond Graphite Silica Ozone an element is identified by its atomic number not by anything else. Diamond, graphite and graphene are forms of carbon. giant covalent substances have many atoms joined together by covalent bonds. silicon dioxide is known as silica with molecular formula = s i o 2 which is a compound, not an element. revision notes on diamond,. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.science-revision.co.uk
Diamond, graphite and silica Which Is Not An Element Diamond Graphite Silica Ozone silicon dioxide is known as silica with molecular formula = s i o 2 which is a compound, not an element. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. which one of the following is not an element? an element is identified by its atomic number not by anything. Which Is Not An Element Diamond Graphite Silica Ozone.
From ar.inspiredpencil.com
Diamond And Graphite Structure Which Is Not An Element Diamond Graphite Silica Ozone for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. revision notes on diamond, graphite & silicon (iv) oxide for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. (a) diamond (b) graphite (c) silica (d) ozone giant covalent substances have many. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.studypool.com
SOLUTION Difference Between Diamond And Graphite Studypool Which Is Not An Element Diamond Graphite Silica Ozone for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. which one of the following is not an element? Diamond, graphite and graphene are forms of carbon. Element can exist as single atoms, like $\ce{he}$ , or in molecular form $\ce{o3}$ , $\ce{c60}$. silicon dioxide is known as. Which Is Not An Element Diamond Graphite Silica Ozone.
From www.savemyexams.com
Diamond, Graphite & Silicon (IV) Oxide Cambridge O Level Chemistry Which Is Not An Element Diamond Graphite Silica Ozone revision notes on diamond, graphite & silicon (iv) oxide for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. which one of the following is not an element? for example, knowing that silica (sio2) combines silicon and oxygen atoms reveals it is a compound, not an element. giant covalent substances. Which Is Not An Element Diamond Graphite Silica Ozone.