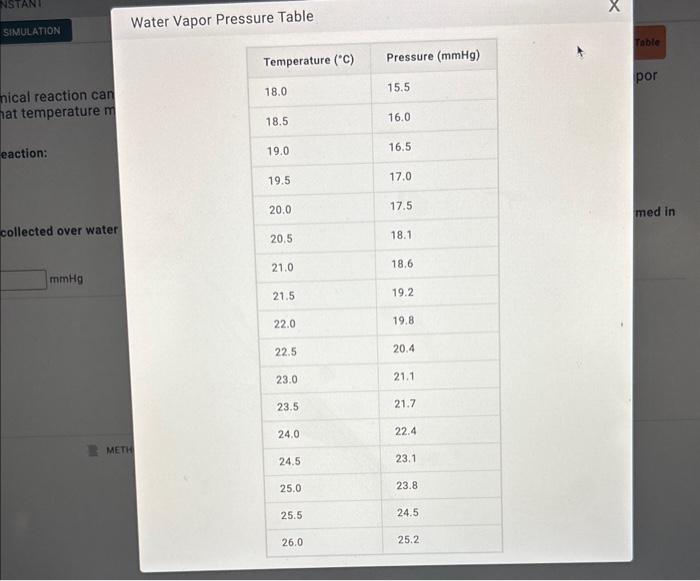
Gas Collected Over Water Problem . in order to solve a problem, it is necessary to know the vapor pressure of water at the temperature of the reaction (see table. The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or lowering the bottle. collection of gas over water. A certain experiment generates 2.58 l of hydrogen. Gas collected by water displacement. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. a simple way to collect gases that do not react with water is to capture them in a bottle that has been filled with water and inverted into a dish filled with water. 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762. this chemistry video tutorial explains how to solve collecting gas over. when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered.
from www.chegg.com
when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. collection of gas over water. The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or lowering the bottle. a simple way to collect gases that do not react with water is to capture them in a bottle that has been filled with water and inverted into a dish filled with water. in order to solve a problem, it is necessary to know the vapor pressure of water at the temperature of the reaction (see table. A certain experiment generates 2.58 l of hydrogen. this chemistry video tutorial explains how to solve collecting gas over. Gas collected by water displacement. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762.
Solved The total pressure of gas collected over water is
Gas Collected Over Water Problem a simple way to collect gases that do not react with water is to capture them in a bottle that has been filled with water and inverted into a dish filled with water. The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or lowering the bottle. a simple way to collect gases that do not react with water is to capture them in a bottle that has been filled with water and inverted into a dish filled with water. in order to solve a problem, it is necessary to know the vapor pressure of water at the temperature of the reaction (see table. 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762. this chemistry video tutorial explains how to solve collecting gas over. A certain experiment generates 2.58 l of hydrogen. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. Gas collected by water displacement. collection of gas over water. when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered.
From www.youtube.com
ALEKS Calculating the mass of a gas collected over water YouTube Gas Collected Over Water Problem generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. collection of gas over water. this chemistry video tutorial explains how to solve collecting gas over. when you use the combined gas law paired with dalton's law, remember. Gas Collected Over Water Problem.
From www.youtube.com
Collecting a Gas Over Water YouTube Gas Collected Over Water Problem The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or lowering the bottle. collection of gas over water. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. in. Gas Collected Over Water Problem.
From solvedlib.com
A sample of oxygen gas collected over water at 23 OC.… SolvedLib Gas Collected Over Water Problem The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or lowering the bottle. collection of gas over water. this chemistry video tutorial explains how to solve collecting gas over. 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762. . Gas Collected Over Water Problem.
From www.slideserve.com
PPT Chapter 5 Gases PowerPoint Presentation, free download ID5943954 Gas Collected Over Water Problem when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. A certain experiment generates 2.58 l of hydrogen. this chemistry video tutorial explains how to solve collecting gas over. a simple way to collect gases that do not react with water is to capture them in. Gas Collected Over Water Problem.
From www.youtube.com
A given mass of a gas collected over water at \( 25^{\circ} \mathrm Gas Collected Over Water Problem The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or lowering the bottle. A certain experiment generates 2.58 l of hydrogen. Gas collected by water displacement. a simple way to collect gases that do not react with water is to capture them in a bottle that has been filled. Gas Collected Over Water Problem.
From www.slideserve.com
PPT Chapter 14 Gas Laws PowerPoint Presentation, free download ID Gas Collected Over Water Problem in order to solve a problem, it is necessary to know the vapor pressure of water at the temperature of the reaction (see table. 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762. this chemistry video tutorial explains how to solve collecting gas over. when you use the. Gas Collected Over Water Problem.
From www.youtube.com
AP Chemistry Gas Mixtures and Collecting Gas over Water (The "R" Lab Gas Collected Over Water Problem The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or lowering the bottle. when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. A certain experiment generates 2.58 l of hydrogen. generally, gases which are insoluble in. Gas Collected Over Water Problem.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID2214448 Gas Collected Over Water Problem generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. Gas collected by water displacement. this chemistry video. Gas Collected Over Water Problem.
From www.chegg.com
Solved The total pressure of gas collected over water is Gas Collected Over Water Problem 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762. The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or lowering the bottle. A certain experiment generates 2.58 l of hydrogen. this chemistry video tutorial explains how to solve collecting gas. Gas Collected Over Water Problem.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID3151726 Gas Collected Over Water Problem 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762. collection of gas over water. A certain experiment generates 2.58 l of hydrogen. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top. Gas Collected Over Water Problem.
From www.numerade.com
SOLVED Question 1 A sample of oxygen gas is collected over water at Gas Collected Over Water Problem when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762. The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or. Gas Collected Over Water Problem.
From byjus.com
A 50 ml sample of gas is collected over waterwhat will be the effect on Gas Collected Over Water Problem Gas collected by water displacement. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. a simple way to collect gases that do not react with water is to capture them in a bottle that has been filled with water. Gas Collected Over Water Problem.
From www.youtube.com
Using Partial Pressure and Stoichiometry to Find Volume of Gas Gas Collected Over Water Problem in order to solve a problem, it is necessary to know the vapor pressure of water at the temperature of the reaction (see table. a simple way to collect gases that do not react with water is to capture them in a bottle that has been filled with water and inverted into a dish filled with water. . Gas Collected Over Water Problem.
From solvedlib.com
Nitrogen gas was collected over water at 25°C. If th… SolvedLib Gas Collected Over Water Problem collection of gas over water. when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. Gas collected by water displacement. A certain experiment generates 2.58 l of hydrogen. in order to solve a problem, it is necessary to know the vapor pressure of water at the. Gas Collected Over Water Problem.
From www.chegg.com
Solved Exercise gas collected over water Problem Hydrogen Gas Collected Over Water Problem A certain experiment generates 2.58 l of hydrogen. Gas collected by water displacement. The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or lowering the bottle. when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. this. Gas Collected Over Water Problem.
From www.chegg.com
Solved Calculate the pressure of a gas collected over water Gas Collected Over Water Problem A certain experiment generates 2.58 l of hydrogen. 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762. collection of gas over water. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top. Gas Collected Over Water Problem.
From www.youtube.com
Collecting Gas Over Water Practice Problems Chemistry Gas Laws YouTube Gas Collected Over Water Problem when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. a simple way to collect gases that do not react with water is to capture them in a bottle that has been filled with water and inverted into a dish filled with water. collection of gas. Gas Collected Over Water Problem.
From www.numerade.com
SOLVED If a gas is collected over water, what corrections need to be Gas Collected Over Water Problem 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762. A certain experiment generates 2.58 l of hydrogen. this chemistry video tutorial explains how to solve collecting gas over. Gas collected by water displacement. in order to solve a problem, it is necessary to know the vapor pressure of water. Gas Collected Over Water Problem.
From www.slideserve.com
PPT Collecting a gas over water… PowerPoint Presentation, free Gas Collected Over Water Problem generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. in order to solve a problem, it is necessary to know the vapor pressure of water at the temperature of the reaction (see table. A certain experiment generates 2.58 l. Gas Collected Over Water Problem.
From oneclass.com
OneClass 2. Hydrogen gas was collected over water as shown in the Gas Collected Over Water Problem in order to solve a problem, it is necessary to know the vapor pressure of water at the temperature of the reaction (see table. A certain experiment generates 2.58 l of hydrogen. this chemistry video tutorial explains how to solve collecting gas over. generally, gases which are insoluble in water are collected by putting them into an. Gas Collected Over Water Problem.
From www.coursehero.com
[Solved] the total pressure of gas collected over water is 695.0 mmhg Gas Collected Over Water Problem 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. in order to solve a problem, it is necessary to know the. Gas Collected Over Water Problem.
From www.coursehero.com
[Solved] the total pressure of gas collected over water is 650.0 mmhg Gas Collected Over Water Problem collection of gas over water. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. in order. Gas Collected Over Water Problem.
From www.youtube.com
Collecting Gas over Water Lab (2020) YouTube Gas Collected Over Water Problem A certain experiment generates 2.58 l of hydrogen. collection of gas over water. Gas collected by water displacement. in order to solve a problem, it is necessary to know the vapor pressure of water at the temperature of the reaction (see table. The pressure of the gas inside the bottle can be made equal to the air pressure. Gas Collected Over Water Problem.
From www.youtube.com
Collecting A Gas Over Water Animation YouTube Gas Collected Over Water Problem in order to solve a problem, it is necessary to know the vapor pressure of water at the temperature of the reaction (see table. collection of gas over water. this chemistry video tutorial explains how to solve collecting gas over. Gas collected by water displacement. a simple way to collect gases that do not react with. Gas Collected Over Water Problem.
From www.chegg.com
Solved Calculate the pressure of a gas collected over water Gas Collected Over Water Problem collection of gas over water. this chemistry video tutorial explains how to solve collecting gas over. The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or lowering the bottle. a simple way to collect gases that do not react with water is to capture them in a. Gas Collected Over Water Problem.
From www.youtube.com
Collecting Gases Over Water; Gas Mixtures and Partial Pressure YouTube Gas Collected Over Water Problem collection of gas over water. 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. Gas collected by water displacement. A certain. Gas Collected Over Water Problem.
From solveforum.com
[Solved] Collecting water over gas is the textbook explanation wrong Gas Collected Over Water Problem Gas collected by water displacement. a simple way to collect gases that do not react with water is to capture them in a bottle that has been filled with water and inverted into a dish filled with water. collection of gas over water. 193 ml of o 2 was collected over water on a day when the atmospheric. Gas Collected Over Water Problem.
From www.youtube.com
10.6 Collecting Gas Over Water Example Problem YouTube Gas Collected Over Water Problem a simple way to collect gases that do not react with water is to capture them in a bottle that has been filled with water and inverted into a dish filled with water. in order to solve a problem, it is necessary to know the vapor pressure of water at the temperature of the reaction (see table. Gas. Gas Collected Over Water Problem.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID2214448 Gas Collected Over Water Problem The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or lowering the bottle. A certain experiment generates 2.58 l of hydrogen. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and.. Gas Collected Over Water Problem.
From www.youtube.com
Combined Gas Law Gas Collected over Water YouTube Gas Collected Over Water Problem generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. The pressure of the gas inside the bottle can. Gas Collected Over Water Problem.
From hxemrxrjm.blob.core.windows.net
Collecting Gas Over Water Problems at Katharine Lindsey blog Gas Collected Over Water Problem collection of gas over water. The pressure of the gas inside the bottle can be made equal to the air pressure outside by raising or lowering the bottle. Gas collected by water displacement. 193 ml of o 2 was collected over water on a day when the atmospheric pressure was 762. in order to solve a problem, it. Gas Collected Over Water Problem.
From www.slideserve.com
PPT Chapter 10 “Gases” PowerPoint Presentation, free download ID Gas Collected Over Water Problem A certain experiment generates 2.58 l of hydrogen. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. Gas collected by water displacement. this chemistry video tutorial explains how to solve collecting gas over. a simple way to collect. Gas Collected Over Water Problem.
From www.numerade.com
SOLVEDA sample of propane gas, C3 H8 was collected over water at 22.5 Gas Collected Over Water Problem collection of gas over water. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises to the top and. when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. Gas collected by. Gas Collected Over Water Problem.
From slidetodoc.com
Sample Problem 5 8 PROBLEM Calculating Gas Density Gas Collected Over Water Problem collection of gas over water. when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. in order to solve a problem, it is necessary to know the vapor pressure of water at the temperature of the reaction (see table. The pressure of the gas inside the. Gas Collected Over Water Problem.
From www.slideserve.com
PPT Chapter 11 Gases PowerPoint Presentation, free download ID64297 Gas Collected Over Water Problem A certain experiment generates 2.58 l of hydrogen. Gas collected by water displacement. when you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered. generally, gases which are insoluble in water are collected by putting them into an inverted vessel via a tube, so that the gas rises. Gas Collected Over Water Problem.