Cu Charge In Cuso4 . The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. Therefore, cuso 4 is a strong electrolyte. We can perform electrolysis of. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water.
from askfilo.com
Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. Therefore, cuso 4 is a strong electrolyte. We can perform electrolysis of. As cuso 4 is an electrolyte, it splits into cu + + (cation) and.
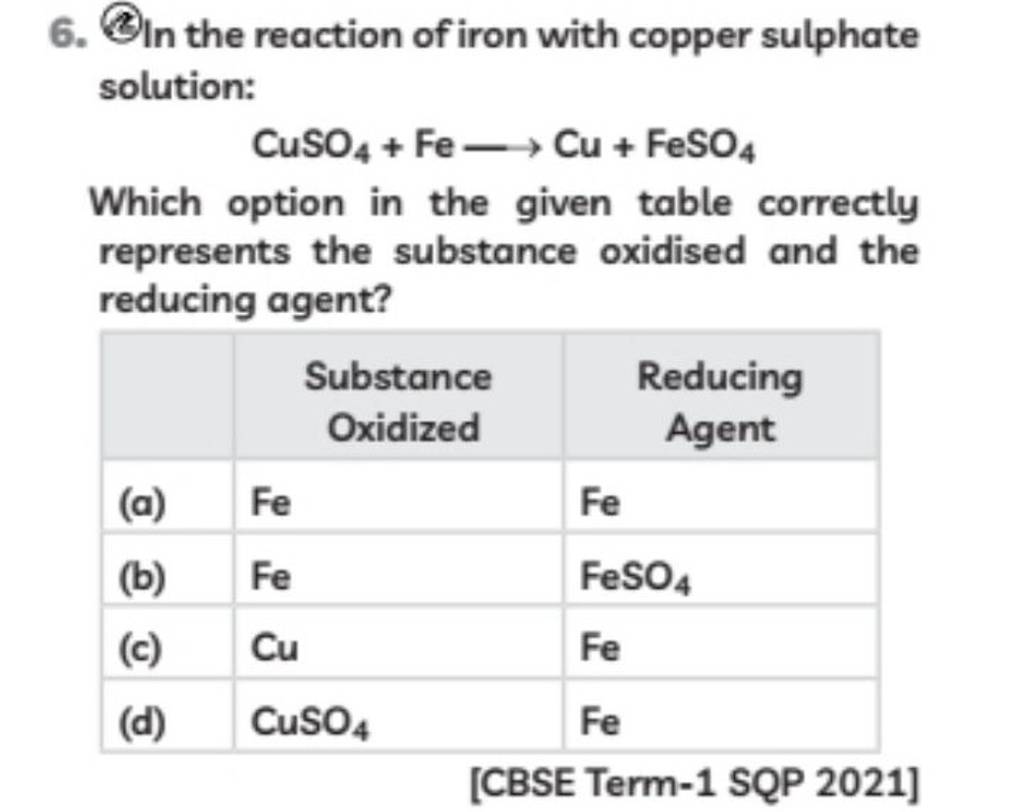
6. In the reaction of iron with copper sulphate solution CuSO4 +Fe Cu+Fe..
Cu Charge In Cuso4 We can perform electrolysis of. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. We can perform electrolysis of. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. Therefore, cuso 4 is a strong electrolyte.
From www.youtube.com
How to balance Fe+CuSo4 =Cu+FeSO4 Chemical equation Al+CuSO4=Al2(SO4)3 Cu Charge In Cuso4 Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid. Cu Charge In Cuso4.
From www.researchgate.net
Fastcharge testing of the Cu0.5 M CuSO4SeC coin cells ac The Cu Charge In Cuso4 This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. We can perform electrolysis of. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. Therefore, cuso 4 is. Cu Charge In Cuso4.
From www.youtube.com
Fe + CuSO4 = FeSO4 + Cu Chemistry YouTube Cu Charge In Cuso4 Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. We can perform electrolysis of. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate. Cu Charge In Cuso4.
From www.meritnation.com
The no Of electron involved in the electro deposition of 63 5gm of Cu Cu Charge In Cuso4 Therefore, cuso 4 is a strong electrolyte. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. To find the correct oxidation state of s in cuso4. Cu Charge In Cuso4.
From www.nagwa.com
Question Video Selecting the Best Choice of Aqueous Solution and Cu Charge In Cuso4 The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. This. Cu Charge In Cuso4.
From byjus.com
Electrolysis of cuso4 solution using copper as electrode Cu Charge In Cuso4 This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. Therefore, cuso 4 is a strong electrolyte. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺). Cu Charge In Cuso4.
From w20.b2m.cz
Al Cuso4 Al2 So4 3 Cu EDUCA Cu Charge In Cuso4 The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. This arrangement allows the copper ion to interact with the sulfate ions, forming. Cu Charge In Cuso4.
From www.toppr.com
1 coulomb of charge passes through solution of AgNO, and CuSO4 Cu Charge In Cuso4 This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. We can perform electrolysis of. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. Therefore, cuso 4 is a strong electrolyte. As cuso 4 is an electrolyte, it splits into cu + + (cation). Cu Charge In Cuso4.
From slideplayer.com
Categorizing Chemical Reactions ppt download Cu Charge In Cuso4 Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. This arrangement allows the copper ion to interact with the sulfate ions,. Cu Charge In Cuso4.
From byjus.com
28.One coulomb of charge is passed through a solution of AgNO3 and Cu Charge In Cuso4 Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. We can perform electrolysis of. Therefore, cuso 4 is a strong electrolyte. This arrangement allows. Cu Charge In Cuso4.
From www.youtube.com
How to Balance CuSO4•5H2O = CuSO4 + H2O YouTube Cu Charge In Cuso4 Therefore, cuso 4 is a strong electrolyte. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. We can perform electrolysis of. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. The. Cu Charge In Cuso4.
From www.chegg.com
Solved What type of reaction is CuSO4(aq) + Zn(s) → Cu(s) + Cu Charge In Cuso4 The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. This arrangement allows the copper ion to interact with the sulfate ions, forming. Cu Charge In Cuso4.
From brainly.com
CuSO4 (aq) + Zn(s) →→→ Cu(s) + ZnSO4 (aq)The singlereplacement Cu Charge In Cuso4 The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. As cuso 4 is an electrolyte, it splits into cu + + (cation) and.. Cu Charge In Cuso4.
From www.researchgate.net
XAS measurements on Cu L and O Kedges. Left panel displays the Cu Cu Charge In Cuso4 The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. As cuso 4 is an electrolyte, it splits into cu + + (cation) and.. Cu Charge In Cuso4.
From www.youtube.com
How to Balance Zn + CuSO4 = Cu + ZnSO4 (Zinc plus Copper (II) Sulfate Cu Charge In Cuso4 Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. We can perform electrolysis of. Therefore, cuso 4 is a strong electrolyte.. Cu Charge In Cuso4.
From gbu-presnenskij.ru
Cuso4 Ionic Charge Save Money gbupresnenskij.ru Cu Charge In Cuso4 The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. As cuso 4 is an electrolyte, it splits into cu + + (cation) and.. Cu Charge In Cuso4.
From www.youtube.com
Fe + CuSO4 gives FeSO4 + Cu (Single Replacement) YouTube Cu Charge In Cuso4 As cuso 4 is an electrolyte, it splits into cu + + (cation) and. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. We can perform electrolysis of. The structure of copper (ii) sulfate,. Cu Charge In Cuso4.
From gbu-presnenskij.ru
Cuso4 Ionic Charge Save Money gbupresnenskij.ru Cu Charge In Cuso4 This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. Therefore, cuso 4 is a strong electrolyte. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. We can perform electrolysis. Cu Charge In Cuso4.
From askfilo.com
6. In the reaction of iron with copper sulphate solution CuSO4 +Fe Cu+Fe.. Cu Charge In Cuso4 We can perform electrolysis of. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. This arrangement allows the copper ion to interact with the. Cu Charge In Cuso4.
From margaretweigel.com
Cu 2h2so4 Cuso4 So2 2h2o Margaret Wiegel™. Jul 2023 Cu Charge In Cuso4 As cuso 4 is an electrolyte, it splits into cu + + (cation) and. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. This. Cu Charge In Cuso4.
From www.youtube.com
CuSO4 + Zn = ZnSO4 + Cu Dis[placement reaction Chemistry Class 10 Cu Charge In Cuso4 We can perform electrolysis of. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. Therefore, cuso 4 is. Cu Charge In Cuso4.
From tecnico.aspillagahornauer.cl
Galvanic Cells Chemistry Steps, 43 OFF Cu Charge In Cuso4 The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. We can perform electrolysis of. To find the correct oxidation state of s in cuso4 (copper (ii). Cu Charge In Cuso4.
From www.youtube.com
How to find the Oxidation Number for Cu in CuSO4 · 5H2O YouTube Cu Charge In Cuso4 Therefore, cuso 4 is a strong electrolyte. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻). Cu Charge In Cuso4.
From www.youtube.com
How to find the Oxidation Number for Cu in Cu2SO4 YouTube Cu Charge In Cuso4 Therefore, cuso 4 is a strong electrolyte. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. We can perform electrolysis of. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. This. Cu Charge In Cuso4.
From bertigamas.github.io
Zn Cuso4 Znso4 Cu Brain Cu Charge In Cuso4 This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. We can perform electrolysis of. Therefore, cuso 4 is a strong electrolyte. To. Cu Charge In Cuso4.
From gbu-presnenskij.ru
Cuso4 Ionic Charge Save Money gbupresnenskij.ru Cu Charge In Cuso4 To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and each element in the. Therefore, cuso 4 is a strong electrolyte. We can perform electrolysis of. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a. Cu Charge In Cuso4.
From bertigamas.github.io
Zn Cuso4 Znso4 Cu Brain Cu Charge In Cuso4 Therefore, cuso 4 is a strong electrolyte. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. We can perform electrolysis of. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. To. Cu Charge In Cuso4.
From bertigamas.github.io
Zn Cuso4 Znso4 Cu Brain Cu Charge In Cuso4 We can perform electrolysis of. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. To find the correct oxidation state of s in cuso4 (copper (ii). Cu Charge In Cuso4.
From brainly.in
Which of the following reactions can be predicted based on the graph Cu Charge In Cuso4 Therefore, cuso 4 is a strong electrolyte. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. To find the correct oxidation state. Cu Charge In Cuso4.
From kunduz.com
[ANSWERED] CuSO4 aq Zn s Cu s ZnSO4 aq The single replacement reaction Cu Charge In Cuso4 The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. We can perform electrolysis of. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. This arrangement allows the copper ion to interact with the. Cu Charge In Cuso4.
From byjus.com
What will happen if Cu rod alone is dipped in cuso4 solution Reduction Cu Charge In Cuso4 This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. As cuso 4 is an electrolyte, it splits into cu + + (cation) and. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry.. Cu Charge In Cuso4.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID4435141 Cu Charge In Cuso4 As cuso 4 is an electrolyte, it splits into cu + + (cation) and. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. This. Cu Charge In Cuso4.
From www.youtube.com
Zn+CuSO4=Cu+ZnSO4 balance the displacement reactionmydocumentary838 Cu Charge In Cuso4 This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. To find the correct oxidation state of s in cuso4 (copper (ii) sulfate), and. Cu Charge In Cuso4.
From www.slideserve.com
PPT 9. OxidationReduction PowerPoint Presentation ID1003963 Cu Charge In Cuso4 As cuso 4 is an electrolyte, it splits into cu + + (cation) and. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. Therefore, cuso 4 is a strong electrolyte. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. To find the correct oxidation state. Cu Charge In Cuso4.
From www.youtube.com
Charge for Copper (Cu) YouTube Cu Charge In Cuso4 We can perform electrolysis of. Whenever copper sulfate or cuso 4 is added to water, it gets dissolved in the water. Therefore, cuso 4 is a strong electrolyte. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. The structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central. Cu Charge In Cuso4.