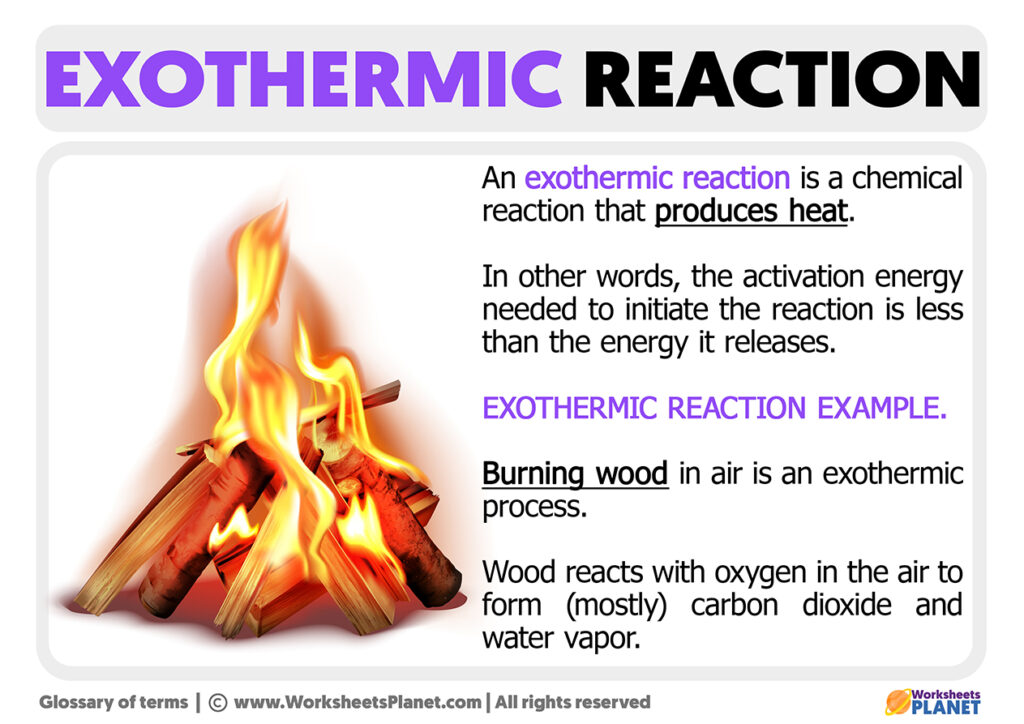
Examples Endothermic And Exothermic Reactions . Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: Examples of endothermic and exothermic processes. A popular example of an endothermic chemical reaction is photosynthesis. Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. On the other hand, an exothermic reaction releases energy into the surrounding of the system. Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting chemistry as well as physics. Photosynthesis is an example of an endothermic chemical reaction. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs.
from
Because heat is absorbed, endothermic reactions feel cold. A popular example of an endothermic chemical reaction is photosynthesis. Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting chemistry as well as physics. Photosynthesis is an example of an endothermic chemical reaction. Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. On the other hand, an exothermic reaction releases energy into the surrounding of the system.
Examples Endothermic And Exothermic Reactions Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. Photosynthesis is an example of an endothermic chemical reaction. Some examples of endothermic reactions are: Because heat is absorbed, endothermic reactions feel cold. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: On the other hand, an exothermic reaction releases energy into the surrounding of the system. A popular example of an endothermic chemical reaction is photosynthesis. Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting chemistry as well as physics. Examples of endothermic and exothermic processes. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. The reaction between ethanoic acid and sodium carbonate. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product.
From
Examples Endothermic And Exothermic Reactions In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting chemistry as well as physics. Photosynthesis is an example of an endothermic chemical reaction. Some examples of endothermic reactions are: Because heat is absorbed, endothermic reactions feel cold. A popular. Examples Endothermic And Exothermic Reactions.
From signalticket9.pythonanywhere.com
Simple Endothermic Reactions With Equations Reaction Balanced Equation Examples Endothermic And Exothermic Reactions Examples of endothermic and exothermic processes. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: Because heat is. Examples Endothermic And Exothermic Reactions.
From 5differencebetween.com
5 Difference Between Endothermic and Exothermic Reaction Endothermic Examples Endothermic And Exothermic Reactions In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Examples of endothermic and exothermic processes. On the other hand, an exothermic reaction releases energy into the surrounding of the system. Photosynthesis is an example of an endothermic. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting chemistry as well as physics. The reaction between ethanoic acid and sodium carbonate. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. On the other hand, an exothermic reaction releases energy into the surrounding of the system.. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. The reaction between ethanoic acid and sodium carbonate. Because heat is absorbed, endothermic reactions feel cold. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. In this process, plants use the energy from the sun to convert. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Examples of endothermic and exothermic processes. The reaction between ethanoic acid and sodium carbonate. Because heat is absorbed, endothermic reactions feel cold. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting chemistry as well as physics. Some. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Some examples of endothermic reactions are: Because heat is absorbed, endothermic reactions feel cold. Examples of endothermic and exothermic processes. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Endothermic and exothermic reactions can be. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Photosynthesis is an example of an endothermic chemical reaction. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. A popular example of an endothermic chemical reaction is photosynthesis. Some examples of endothermic reactions are: Examples of endothermic and exothermic processes. This reaction requires 15mj of energy (sunlight) for every. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. Examples of endothermic and exothermic processes. On the other hand, an exothermic reaction releases energy into the surrounding of the system. Endothermic and exothermic reactions can. Examples Endothermic And Exothermic Reactions.
From question.pandai.org
Endothermic and exothermic reactions Examples Endothermic And Exothermic Reactions On the other hand, an exothermic reaction releases energy into the surrounding of the system. This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: Examples of endothermic and exothermic processes. Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting chemistry as well as physics. Photosynthesis is an example of an endothermic chemical reaction.. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Photosynthesis is an example of an endothermic chemical reaction. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting chemistry as well as physics. This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: A popular example. Examples Endothermic And Exothermic Reactions.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Examples Endothermic And Exothermic Reactions This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. A popular example of an endothermic chemical reaction is photosynthesis. Examples of endothermic and exothermic processes. Photosynthesis is an example of an endothermic chemical reaction. Examples of. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Because heat is absorbed, endothermic reactions feel cold. On the other hand, an exothermic reaction releases energy into the surrounding of the system. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. Some examples of. Examples Endothermic And Exothermic Reactions.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Examples Endothermic And Exothermic Reactions Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. Photosynthesis is an example of an endothermic chemical reaction. Examples of endothermic and exothermic processes. In this process, plants use the. Examples Endothermic And Exothermic Reactions.
From www.pinterest.it
Endothermic and Exothermic Reactions Lab ⋆ Exothermic Examples Endothermic And Exothermic Reactions On the other hand, an exothermic reaction releases energy into the surrounding of the system. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Examples of endothermic and exothermic processes. Photosynthesis is an example of an endothermic chemical reaction. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. On the other hand, an exothermic reaction releases energy into the surrounding of the system. Because heat is absorbed, endothermic reactions feel cold. A popular example of an endothermic chemical reaction is photosynthesis. Photosynthesis is an example of an endothermic. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. The reaction between ethanoic acid and sodium carbonate. This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: A popular example of an endothermic chemical reaction is photosynthesis. Some examples of endothermic reactions are: Exothermic reactions range. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Because heat is absorbed, endothermic reactions feel cold. Photosynthesis is an example of an endothermic chemical reaction. On the other hand, an exothermic reaction releases. Examples Endothermic And Exothermic Reactions.
From narodnatribuna.info
Endothermic Reactions Definition And Examples Examples Endothermic And Exothermic Reactions On the other hand, an exothermic reaction releases energy into the surrounding of the system. Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting chemistry as well as physics. Because heat is absorbed, endothermic reactions feel cold. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen.. Examples Endothermic And Exothermic Reactions.
From mungfali.com
Exothermic Vs Endothermic Diagram Examples Endothermic And Exothermic Reactions Because heat is absorbed, endothermic reactions feel cold. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Some examples of endothermic reactions are: An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The reaction between ethanoic acid and sodium carbonate. Examples of endothermic and exothermic processes. In simple terms, the. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions The reaction between ethanoic acid and sodium carbonate. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Some examples of endothermic reactions are: This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions A popular example of an endothermic chemical reaction is photosynthesis. The reaction between ethanoic acid and sodium carbonate. Examples of endothermic and exothermic processes. This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. An endothermic reaction. Examples Endothermic And Exothermic Reactions.
From studymind.co.uk
Endothermic vs Exothermic Reactions (GCSE Chemistry) Study Mind Examples Endothermic And Exothermic Reactions In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Examples of endothermic reactions include photosynthesis, dissolving. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions On the other hand, an exothermic reaction releases energy into the surrounding of the system. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Examples of endothermic and exothermic processes. Because heat is absorbed, endothermic reactions feel cold. In simple terms, the endothermic reactions absorb energy from the. Examples Endothermic And Exothermic Reactions.
From www.slideserve.com
PPT Energy Changes PowerPoint Presentation, free download ID3470966 Examples Endothermic And Exothermic Reactions On the other hand, an exothermic reaction releases energy into the surrounding of the system. Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Examples of endothermic and exothermic processes. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. A popular example of an endothermic chemical reaction is photosynthesis. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting. Examples Endothermic And Exothermic Reactions.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Examples Endothermic And Exothermic Reactions On the other hand, an exothermic reaction releases energy into the surrounding of the system. This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: Because heat is absorbed, endothermic reactions feel cold. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Endothermic and exothermic reactions can be thought of as having. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions The reaction between ethanoic acid and sodium carbonate. Because heat is absorbed, endothermic reactions feel cold. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. In simple terms,. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Photosynthesis is an example of an endothermic chemical reaction. Some examples of endothermic reactions are: This reaction requires 15mj of energy (sunlight) for every kilogram of glucose produced: Because heat is absorbed, endothermic reactions feel cold. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Exothermic reactions range. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting chemistry as well as physics. The reaction between ethanoic acid and sodium carbonate. Photosynthesis is an example of an endothermic chemical reaction. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. Examples of endothermic and exothermic processes.. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Examples of endothermic and exothermic processes. Photosynthesis is an example of an endothermic chemical reaction. A popular example of an endothermic chemical reaction is photosynthesis. Some examples of endothermic reactions are: An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Because heat is absorbed, endothermic reactions feel cold. Some examples of endothermic reactions are: Exothermic reactions range from safe, gentle to dramatic, explosive and involve interesting chemistry as well as physics. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In this process, plants use the energy from the sun to convert carbon dioxide and. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Photosynthesis is an example of an endothermic chemical reaction. Because heat is absorbed, endothermic reactions feel cold. The reaction between ethanoic acid and sodium carbonate. Some examples of endothermic reactions are: A popular example of an endothermic chemical reaction is photosynthesis. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Exothermic reactions range from safe,. Examples Endothermic And Exothermic Reactions.
From fixdiagramzoolatrous.z21.web.core.windows.net
Enthalpy Diagram For Endothermic Reaction Examples Endothermic And Exothermic Reactions A popular example of an endothermic chemical reaction is photosynthesis. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The reaction between ethanoic acid and sodium carbonate. On the other hand, an exothermic reaction releases energy into the surrounding of the system. In this process, plants use the energy from the sun to convert carbon. Examples Endothermic And Exothermic Reactions.
From
Examples Endothermic And Exothermic Reactions Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. On the other hand, an exothermic reaction releases energy into the surrounding of the system. Examples of endothermic and exothermic processes. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Photosynthesis is an example of an endothermic chemical reaction. In simple. Examples Endothermic And Exothermic Reactions.