Evaporation Pressure Temperature Relationship . It is an intrinsic property that is a function of temperature alone. It is important to note that when a. Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. It is the pressure at which gas is in thermodynamic equilibrium with its condensed state. The pressure at this point is known as the saturated vapor pressure. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Evaporation, vapor pressure, and boiling point. An equilibrium point is reached when the evaporation rate equals that of condensation. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of. If the container is closed, an equilibrium is reached where an equal. Explain the relationship between relative humidity and partial pressure of water vapor. Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular).
from www.engineeringtoolbox.com
If the container is closed, an equilibrium is reached where an equal. Explain the relationship between relative humidity and partial pressure of water vapor. Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of. Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. The pressure at this point is known as the saturated vapor pressure. Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: An equilibrium point is reached when the evaporation rate equals that of condensation. It is important to note that when a.
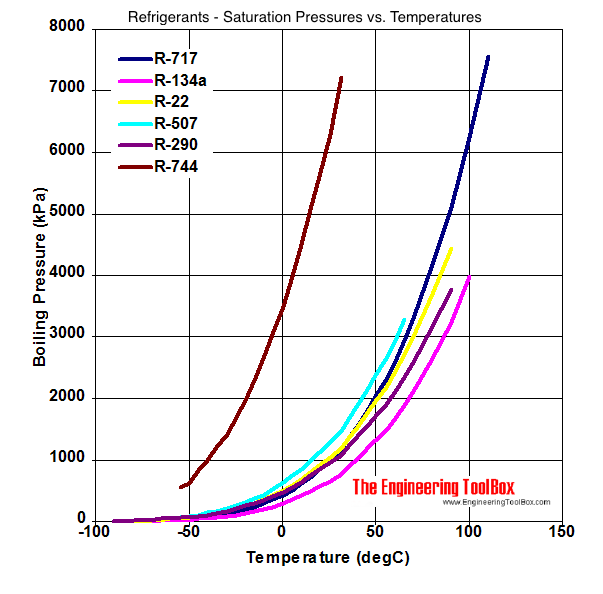
Refrigerants Temperature and Pressure at Constant Boiling
Evaporation Pressure Temperature Relationship Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Evaporation, vapor pressure, and boiling point. It is an intrinsic property that is a function of temperature alone. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Explain the relationship between relative humidity and partial pressure of water vapor. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: It is important to note that when a. It is the pressure at which gas is in thermodynamic equilibrium with its condensed state. If the container is closed, an equilibrium is reached where an equal. An equilibrium point is reached when the evaporation rate equals that of condensation. Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of. The pressure at this point is known as the saturated vapor pressure. Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor.
From hvacrschool.com
Saturation and the PressureTemperature Relationship HVAC School Evaporation Pressure Temperature Relationship Explain the relationship between relative humidity and partial pressure of water vapor. Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of. The pressure at this point is known as the saturated vapor pressure. It is an intrinsic property that is a function of temperature alone. This equation. Evaporation Pressure Temperature Relationship.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Evaporation Pressure Temperature Relationship Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: It is an intrinsic property that is a function of temperature alone. The pressure at this point is known as the saturated vapor pressure. It is the pressure at which gas is in thermodynamic equilibrium with its condensed state. This equation can be used. Evaporation Pressure Temperature Relationship.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Evaporation Pressure Temperature Relationship Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. If the container is closed,. Evaporation Pressure Temperature Relationship.
From pharmacyscope.com
Vapour pressure Pharmacy Scope Evaporation Pressure Temperature Relationship Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of. If the container is closed, an equilibrium is reached where an equal. The pressure at this point is known as the saturated vapor pressure. It is the pressure at which gas is in thermodynamic equilibrium with its condensed. Evaporation Pressure Temperature Relationship.
From socratic.org
Which graph shows the relationship between the temperature and volume Evaporation Pressure Temperature Relationship It is an intrinsic property that is a function of temperature alone. Evaporation, vapor pressure, and boiling point. It is the pressure at which gas is in thermodynamic equilibrium with its condensed state. Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Enter the pressure and temperature in any of five units. Evaporation Pressure Temperature Relationship.
From socratic.org
What is the relation between critical temperature and boiling point or Evaporation Pressure Temperature Relationship Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). Evaporation, vapor pressure, and boiling point. An equilibrium point is reached when the evaporation rate equals that of condensation. It is an intrinsic property that is a function of temperature alone. It is the pressure at which gas is in thermodynamic equilibrium with its condensed state.. Evaporation Pressure Temperature Relationship.
From www.researchgate.net
For two different evaporation temperatures the dependence of the Evaporation Pressure Temperature Relationship This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Explain the relationship between relative humidity and partial pressure of water vapor. It is an intrinsic property that is a function of temperature alone. Vapor pressures have an exponential relationship with temperature and always increase as. Evaporation Pressure Temperature Relationship.
From courses.lumenlearning.com
Phase Diagrams Chemistry for Majors Evaporation Pressure Temperature Relationship An equilibrium point is reached when the evaporation rate equals that of condensation. The pressure at this point is known as the saturated vapor pressure. Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). It is important to note that when a. It is an intrinsic property that is a function of temperature alone. This. Evaporation Pressure Temperature Relationship.
From mungfali.com
Pressure Temperature Relationship Chart Evaporation Pressure Temperature Relationship It is important to note that when a. An equilibrium point is reached when the evaporation rate equals that of condensation. Explain the relationship between relative humidity and partial pressure of water vapor. Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of. Explain the relationship between vapor. Evaporation Pressure Temperature Relationship.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Evaporation Pressure Temperature Relationship The pressure at this point is known as the saturated vapor pressure. It is important to note that when a. If the container is closed, an equilibrium is reached where an equal. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: It is an intrinsic property that is a function of temperature alone.. Evaporation Pressure Temperature Relationship.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Evaporation Pressure Temperature Relationship An equilibrium point is reached when the evaporation rate equals that of condensation. It is an intrinsic property that is a function of temperature alone. Evaporation, vapor pressure, and boiling point. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: It is the pressure at which gas is in thermodynamic equilibrium with its. Evaporation Pressure Temperature Relationship.
From courses.lumenlearning.com
Phase Changes Physics Evaporation Pressure Temperature Relationship Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of. Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: It is an intrinsic property that is a. Evaporation Pressure Temperature Relationship.
From www.engineeringtoolbox.com
Refrigerants Temperature and Pressure at Constant Boiling Evaporation Pressure Temperature Relationship Evaporation, vapor pressure, and boiling point. The pressure at this point is known as the saturated vapor pressure. Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. It is the pressure at which gas is in thermodynamic equilibrium with its condensed state. Enter the pressure and temperature in any of five units. Evaporation Pressure Temperature Relationship.
From www.researchgate.net
Effect of evaporation temperature on performances of dual pressure Evaporation Pressure Temperature Relationship Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Evaporation, vapor pressure, and boiling point. It is an intrinsic property that is a function of temperature alone. An equilibrium point is reached when the evaporation rate equals that of condensation. If the container is closed, an equilibrium is reached where an equal. Enter. Evaporation Pressure Temperature Relationship.
From www.researchgate.net
Operating pressure and evaporation temperature as a function of the Evaporation Pressure Temperature Relationship If the container is closed, an equilibrium is reached where an equal. It is important to note that when a. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. The pressure at this point is known as the saturated vapor pressure. Explain the relationship between. Evaporation Pressure Temperature Relationship.
From www.researchgate.net
Temperature, pressure, and depth relationships. Download Scientific Evaporation Pressure Temperature Relationship Evaporation, vapor pressure, and boiling point. It is the pressure at which gas is in thermodynamic equilibrium with its condensed state. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). If the container is closed, an equilibrium is reached. Evaporation Pressure Temperature Relationship.
From www.researchgate.net
The vapor pressure of ethanol vs. the normal boilingpoint temperature Evaporation Pressure Temperature Relationship Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Explain the relationship between relative humidity and partial pressure of water vapor. The pressure at this point is known as the saturated vapor pressure. It is the pressure at which gas is in thermodynamic equilibrium with its condensed state. This equation can be. Evaporation Pressure Temperature Relationship.
From refrigeratorsreviewed.com
TemperaturePressure Relationship In Refrigeration Explained Indepth Evaporation Pressure Temperature Relationship Evaporation, vapor pressure, and boiling point. It is important to note that when a. An equilibrium point is reached when the evaporation rate equals that of condensation. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured. Evaporation Pressure Temperature Relationship.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Evaporation Pressure Temperature Relationship The pressure at this point is known as the saturated vapor pressure. It is an intrinsic property that is a function of temperature alone. Explain the relationship between relative humidity and partial pressure of water vapor. Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Enter the pressure and temperature in any. Evaporation Pressure Temperature Relationship.
From www.youtube.com
Pressure, Volume, Temperature and Mole Relationships YouTube Evaporation Pressure Temperature Relationship Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: An equilibrium point is reached when the evaporation rate equals that of condensation. It is important to note that when a. The pressure at this point is known as the saturated vapor pressure. Evaporation, vapor pressure, and boiling point. It is the pressure at. Evaporation Pressure Temperature Relationship.
From www.researchgate.net
Change of heat input with evaporation pressure Download Scientific Evaporation Pressure Temperature Relationship An equilibrium point is reached when the evaporation rate equals that of condensation. Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of. If the container is closed, an equilibrium is reached where an equal. Evaporation, vapor pressure, and boiling point. It is an intrinsic property that is. Evaporation Pressure Temperature Relationship.
From vacaero.com
Evaporation Evaporation Pressure Temperature Relationship Evaporation, vapor pressure, and boiling point. It is important to note that when a. Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of. If the container is closed, an equilibrium is reached where an equal. Both vapor pressure and boiling point are affected by the strength of. Evaporation Pressure Temperature Relationship.
From www.peoi.org
Chapter 10 Section C Properties of Liquids Evaporation Pressure Temperature Relationship Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of. Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. It is the pressure at which gas is in thermodynamic equilibrium with its condensed state. Explain the relationship between relative humidity. Evaporation Pressure Temperature Relationship.
From learningschooltrkesp5v.z22.web.core.windows.net
Water Evaporation Rate By Temperature Evaporation Pressure Temperature Relationship Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). If the container is closed, an equilibrium is reached where an equal. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor. Evaporation Pressure Temperature Relationship.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Evaporation Pressure Temperature Relationship It is the pressure at which gas is in thermodynamic equilibrium with its condensed state. Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). Explain the relationship between relative humidity and partial pressure of water vapor. An equilibrium point is reached when the evaporation rate equals that of condensation. Explain the relationship between vapor pressure. Evaporation Pressure Temperature Relationship.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Evaporation Pressure Temperature Relationship Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. If the container is closed, an equilibrium is reached where an equal. Evaporation, vapor pressure, and boiling point. Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). The pressure at this point is known as the saturated vapor. Evaporation Pressure Temperature Relationship.
From www.researchgate.net
A. Relationship between vapor pressure, relative humidity and Evaporation Pressure Temperature Relationship Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of. Explain the relationship between relative humidity and partial pressure of water vapor. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: This equation can be used to calculate the enthalpy of. Evaporation Pressure Temperature Relationship.
From scienceline.ucsb.edu
UCSB Science Line Evaporation Pressure Temperature Relationship An equilibrium point is reached when the evaporation rate equals that of condensation. It is important to note that when a. Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. If. Evaporation Pressure Temperature Relationship.
From refrigerants.com
Pressure Temperature Chart National Refrigerants, Inc. Evaporation Pressure Temperature Relationship Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). If the container is closed, an equilibrium is reached where an equal. It is an intrinsic property that is a function of temperature alone. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Explain the relationship between vapor pressure. Evaporation Pressure Temperature Relationship.
From www.researchgate.net
Evolution of water vapor pressure Pvapor along with interfacial Evaporation Pressure Temperature Relationship The pressure at this point is known as the saturated vapor pressure. An equilibrium point is reached when the evaporation rate equals that of condensation. Both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular). This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two. Evaporation Pressure Temperature Relationship.
From www.researchgate.net
Relationship between temperature and pressure. Download Scientific Evaporation Pressure Temperature Relationship Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch, or millimeters of. It is an intrinsic property that is a function of temperature alone. Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. The pressure at this point is known as the. Evaporation Pressure Temperature Relationship.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Evaporation Pressure Temperature Relationship Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: If the container is closed, an equilibrium is reached where an equal. Explain the relationship between relative humidity and partial pressure of water vapor. The pressure at. Evaporation Pressure Temperature Relationship.
From www.researchgate.net
Vapor pressure of methanol and ethanol as a function of temperature Evaporation Pressure Temperature Relationship It is an intrinsic property that is a function of temperature alone. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Evaporation, vapor pressure, and boiling point. Explain the relationship between relative humidity and partial pressure of water vapor. It is the pressure at which. Evaporation Pressure Temperature Relationship.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Evaporation Pressure Temperature Relationship Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Explain the relationship between relative humidity and partial pressure of water vapor. An equilibrium point is reached when the evaporation rate equals that of condensation. Enter the pressure and temperature in any of five units of pressure (atmospheres, bar, kilopascals, pounds per square inch,. Evaporation Pressure Temperature Relationship.
From www.numerade.com
SOLVED Table 10.2 Vapor Pressure of Water at Various Temperatures Evaporation Pressure Temperature Relationship This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Evaporation, vapor pressure, and boiling point. Explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Both vapor pressure and boiling point are affected by the strength of interparticle. Evaporation Pressure Temperature Relationship.