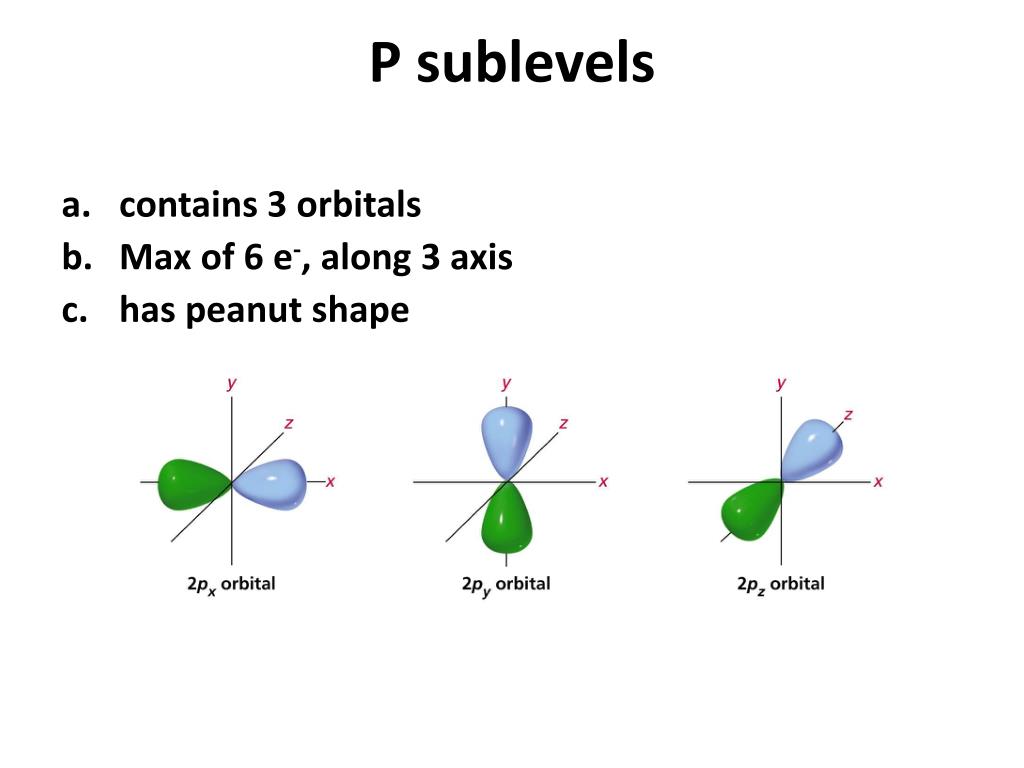
How Many Electrons Can Occupy The 3P Subshell . The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons. A neutral phosphorus atom has 15 electrons. Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2. There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the electron can occupy any one of these p orbitals. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Carbon (atomic number 6) has six electrons. Each atomic orbital can be occupied by a maximum of two electrons; Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p. The third shell can carry up 18 electrons, but it is more stable by carrying only. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. This means that the number of orbitals in each subshell is as follows: One orbital (1 x 2 = total of 2. Electrons are placed into available shells, starting with the lowest energy level. This means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons.
from www.slideserve.com
The third shell can carry up 18 electrons, but it is more stable by carrying only. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons. Carbon (atomic number 6) has six electrons. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. This means that the number of orbitals in each subshell is as follows: One orbital (1 x 2 = total of 2. This means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons. Electrons are placed into available shells, starting with the lowest energy level. Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2.
PPT Electron configurations PowerPoint Presentation, free download
How Many Electrons Can Occupy The 3P Subshell The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons. One orbital (1 x 2 = total of 2. Carbon (atomic number 6) has six electrons. The third shell can carry up 18 electrons, but it is more stable by carrying only. This means that the number of orbitals in each subshell is as follows: Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p. This means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Each atomic orbital can be occupied by a maximum of two electrons; When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the electron can occupy any one of these p orbitals. A neutral phosphorus atom has 15 electrons. The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons. Electrons are placed into available shells, starting with the lowest energy level. Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2.
From chemistryatomrevision.weebly.com
Electron Configuration Atomic Structure How Many Electrons Can Occupy The 3P Subshell The first shell can carry up to two electrons, the second shell can carry up to eight electrons. A neutral phosphorus atom has 15 electrons. The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons. When drawing orbital diagrams, we include empty boxes to depict any empty. How Many Electrons Can Occupy The 3P Subshell.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica How Many Electrons Can Occupy The 3P Subshell This means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are. How Many Electrons Can Occupy The 3P Subshell.
From www.slideserve.com
PPT Sorbitals PowerPoint Presentation, free download ID1830175 How Many Electrons Can Occupy The 3P Subshell Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Each atomic orbital can be occupied by a maximum of two electrons; One orbital (1 x 2 = total of 2. There. How Many Electrons Can Occupy The 3P Subshell.
From chem.libretexts.org
3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts How Many Electrons Can Occupy The 3P Subshell A neutral phosphorus atom has 15 electrons. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p. The third shell can carry up 18 electrons, but it is more stable by carrying only. Each atomic orbital can be occupied by a maximum of two electrons; One orbital (1. How Many Electrons Can Occupy The 3P Subshell.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry How Many Electrons Can Occupy The 3P Subshell The third shell can carry up 18 electrons, but it is more stable by carrying only. There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the electron can occupy any one of these p orbitals. This means that the number of orbitals in each subshell is as follows: Two electrons can go into the 1s subshell,. How Many Electrons Can Occupy The 3P Subshell.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry How Many Electrons Can Occupy The 3P Subshell When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2. The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold. How Many Electrons Can Occupy The 3P Subshell.
From toppr.com
The subshell that arises after f is called g subshell. How many How Many Electrons Can Occupy The 3P Subshell Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p.. How Many Electrons Can Occupy The 3P Subshell.
From icdsc.org
How many electrons are in each shell including 3p orbitals How Many Electrons Can Occupy The 3P Subshell The first shell can carry up to two electrons, the second shell can carry up to eight electrons. One orbital (1 x 2 = total of 2. There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the electron can occupy any one of these p orbitals. Using the above you can work out the maximum number. How Many Electrons Can Occupy The 3P Subshell.
From icdsc.org
How many electrons are in each shell including 3p orbitals How Many Electrons Can Occupy The 3P Subshell One orbital (1 x 2 = total of 2. There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the electron can occupy any one of these p orbitals. Carbon (atomic number 6) has six electrons. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The subshells s,. How Many Electrons Can Occupy The 3P Subshell.
From www.nagwa.com
Question Video Determining the Maximum Number of Electrons in an S How Many Electrons Can Occupy The 3P Subshell One orbital (1 x 2 = total of 2. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Each atomic orbital can be occupied by a maximum of two electrons; Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the. How Many Electrons Can Occupy The 3P Subshell.
From www.slideserve.com
PPT Subshells & orbitals PowerPoint Presentation, free download ID How Many Electrons Can Occupy The 3P Subshell Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2. The third shell can carry up 18 electrons, but it is more stable by carrying only. There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the electron can occupy any one of these p orbitals. The. How Many Electrons Can Occupy The 3P Subshell.
From www.slideshare.net
Mec chapter 2 How Many Electrons Can Occupy The 3P Subshell When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. Electrons are placed into available shells, starting with the lowest energy level. The third shell can carry up 18 electrons, but it is more stable by carrying only. The subshells s, p, d, and f contain the following number. How Many Electrons Can Occupy The 3P Subshell.
From mungfali.com
Electron Subshell Chart How Many Electrons Can Occupy The 3P Subshell When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. One orbital (1 x 2 = total of 2. This means that the number of orbitals in each subshell is as follows: Using the above you can work out the maximum number of electrons that can occupy a shell. How Many Electrons Can Occupy The 3P Subshell.
From www.slideserve.com
PPT Bohr’s model PowerPoint Presentation, free download ID2635508 How Many Electrons Can Occupy The 3P Subshell A neutral phosphorus atom has 15 electrons. One orbital (1 x 2 = total of 2. The third shell can carry up 18 electrons, but it is more stable by carrying only. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. Using the above you can work out. How Many Electrons Can Occupy The 3P Subshell.
From www.thoughtco.com
Electron Configuration Chart How Many Electrons Can Occupy The 3P Subshell There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the electron can occupy any one of these p orbitals. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p. This means that the number of orbitals in each subshell is as follows: The third. How Many Electrons Can Occupy The 3P Subshell.
From www.slideserve.com
PPT Electron Configurations and Orbital Diagrams PowerPoint How Many Electrons Can Occupy The 3P Subshell A neutral phosphorus atom has 15 electrons. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2. One orbital (1 x 2 = total of 2. Carbon (atomic number 6) has six. How Many Electrons Can Occupy The 3P Subshell.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID2664384 How Many Electrons Can Occupy The 3P Subshell This means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons. Each atomic orbital can be occupied by a maximum of two electrons; There are three degenerate 2 p orbitals (ml. How Many Electrons Can Occupy The 3P Subshell.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps How Many Electrons Can Occupy The 3P Subshell The third shell can carry up 18 electrons, but it is more stable by carrying only. The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons. A neutral phosphorus atom has 15 electrons. There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the. How Many Electrons Can Occupy The 3P Subshell.
From www.slideserve.com
PPT Unit 7 Electronic Structure and the Periodic Table PowerPoint How Many Electrons Can Occupy The 3P Subshell Each atomic orbital can be occupied by a maximum of two electrons; The third shell can carry up 18 electrons, but it is more stable by carrying only. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. This means that the number of orbitals in each subshell is as follows: Carbon. How Many Electrons Can Occupy The 3P Subshell.
From www.chegg.com
Solved 10. How many electrons can occupy a subshell How Many Electrons Can Occupy The 3P Subshell When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. The third shell can carry up 18 electrons, but it is more stable by carrying only. This means that the number of orbitals in each subshell is as follows: The first shell can carry up to two electrons, the. How Many Electrons Can Occupy The 3P Subshell.
From www.slideserve.com
PPT Electron Configurations PowerPoint Presentation, free download How Many Electrons Can Occupy The 3P Subshell A neutral phosphorus atom has 15 electrons. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons. This means that the s orbital can contain up to two electrons,. How Many Electrons Can Occupy The 3P Subshell.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube How Many Electrons Can Occupy The 3P Subshell The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2. This means that the number of orbitals in each subshell is as follows: The subshells s, p, d, and f contain the. How Many Electrons Can Occupy The 3P Subshell.
From www.animalia-life.club
Electron Shell Diagram How Many Electrons Can Occupy The 3P Subshell One orbital (1 x 2 = total of 2. A neutral phosphorus atom has 15 electrons. This means that the number of orbitals in each subshell is as follows: The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons. There are three degenerate 2 p orbitals (ml. How Many Electrons Can Occupy The 3P Subshell.
From chem.libretexts.org
8.3 Electron Configurations How Electrons Occupy Orbitals Chemistry How Many Electrons Can Occupy The 3P Subshell Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p. A neutral phosphorus atom has 15 electrons. This means that the number of orbitals in each subshell is as follows: Electrons are placed into available shells, starting with the lowest energy level. The third shell can carry up. How Many Electrons Can Occupy The 3P Subshell.
From mungfali.com
Orbital Subshell Chart How Many Electrons Can Occupy The 3P Subshell Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2. Electrons are placed into available shells, starting with the lowest energy level. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Two electrons can go into the 1s subshell, 2 can. How Many Electrons Can Occupy The 3P Subshell.
From www.slideserve.com
PPT Electron Configurations and Orbital Diagrams PowerPoint How Many Electrons Can Occupy The 3P Subshell This means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons. A neutral phosphorus atom has 15 electrons. There are three degenerate 2 p orbitals (ml = −1, 0, +1) and. How Many Electrons Can Occupy The 3P Subshell.
From www.chegg.com
Solved How many electrons could occupy a subshell with the How Many Electrons Can Occupy The 3P Subshell This means that the number of orbitals in each subshell is as follows: Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p. A neutral phosphorus atom has 15 electrons. Each atomic orbital can be occupied by a maximum of two electrons; This means that the s orbital. How Many Electrons Can Occupy The 3P Subshell.
From www.slideserve.com
PPT Electron configurations PowerPoint Presentation, free download How Many Electrons Can Occupy The 3P Subshell Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2. Carbon (atomic number 6) has six electrons. This means that the number of orbitals in each subshell is as follows: One orbital (1 x 2 = total of 2. This means that the s orbital can contain up to two. How Many Electrons Can Occupy The 3P Subshell.
From www.animalia-life.club
Electron Shell Diagram How Many Electrons Can Occupy The 3P Subshell A neutral phosphorus atom has 15 electrons. Carbon (atomic number 6) has six electrons. This means that the number of orbitals in each subshell is as follows: Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2. Two electrons can go into the 1s subshell, 2 can go into the. How Many Electrons Can Occupy The 3P Subshell.
From saylordotorg.github.io
Building Up the Periodic Table How Many Electrons Can Occupy The 3P Subshell Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p. Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2. A neutral phosphorus atom has 15 electrons. The subshells s, p, d, and f contain the following number. How Many Electrons Can Occupy The 3P Subshell.
From www.slideserve.com
PPT Electron Configurations and the Periodic Table PowerPoint How Many Electrons Can Occupy The 3P Subshell The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Carbon (atomic number 6) has six electrons. The third shell can carry up 18 electrons, but it is more stable by carrying only. There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the electron can occupy any one. How Many Electrons Can Occupy The 3P Subshell.
From www.chegg.com
Solved QUESTION 6 How many electrons occupy the 3p subshell How Many Electrons Can Occupy The 3P Subshell One orbital (1 x 2 = total of 2. Electrons are placed into available shells, starting with the lowest energy level. Each atomic orbital can be occupied by a maximum of two electrons; There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the electron can occupy any one of these p orbitals. The first shell can. How Many Electrons Can Occupy The 3P Subshell.
From chem.libretexts.org
8.3 Electron Configurations How Electrons Occupy Orbitals Chemistry How Many Electrons Can Occupy The 3P Subshell Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the electron can occupy any one of. How Many Electrons Can Occupy The 3P Subshell.
From www.numerade.com
SOLVEDThe subshell that arises after f is called the g subshell. How How Many Electrons Can Occupy The 3P Subshell The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the electron can. How Many Electrons Can Occupy The 3P Subshell.
From courses.lumenlearning.com
Electrons Biology for Majors I How Many Electrons Can Occupy The 3P Subshell A neutral phosphorus atom has 15 electrons. Electrons are placed into available shells, starting with the lowest energy level. There are three degenerate 2 p orbitals (ml = −1, 0, +1) and the electron can occupy any one of these p orbitals. This means that the s orbital can contain up to two electrons, the p orbital can contain up. How Many Electrons Can Occupy The 3P Subshell.